Controversies and challenges in the histologic subtyping of lung adenocarcinoma
Introduction
In the eight years since the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) international multidisciplinary classification of lung adenocarcinoma was published, a considerable amount of data has been generated regarding its validity and applicability (1). This article explores those data and considers potential future modifications to the IASLC/ATS/ERS lung adenocarcinoma classification that will have prognostic impact.
IASLC/ATS/ERS lung adenocarcinoma classification—a paradigm shift
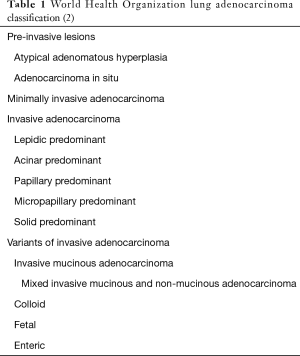
The IASLC/ATS/ERS lung adenocarcinoma classification scheme represented a paradigm shift from preceding World Health Organization (WHO) classifications. Gone were designations such as mixed subtype adenocarcinoma that lacked the granularity necessary to make prognostically meaningful comparisons, and bronchioloalveolar carcinoma (BAC), which in practice, was being assigned imprecisely to a histologically and prognostically diverse array of tumors (1). In their place was a classification that separated preinvasive lesions, specifically atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ (AIS), from invasive adenocarcinomas. Invasive lung adenocarcinomas were for the first time subdivided on the basis of size of the invasive component rather than total tumor size. Minimally invasive adenocarcinoma (MIA) was introduced for tumors with no greater than 5 mm of invasion, while invasive adenocarcinomas were classified according to their predominant histologic pattern into lepidic, acinar, papillary, micropapillary, and solid-predominant categories. Variants of invasive adenocarcinoma, including invasive mucinous, mixed nonmucinous and mucinous, colloid, fetal, and enteric adenocarcinoma were afforded their own categories. Multiple studies have demonstrated that the IASLC/ATS/ERS classification has predictive value, which led to it being incorporated into the current WHO classification (Table 1) (2,3). Among tumors categorized using this classification scheme, AIS and MIA are expected not to recur following complete resection, while lepidic-predominant adenocarcinoma has almost as favorable a prognosis, acinar and papillary-predominant adenocarcinomas are of intermediate recurrence risk, and micropapillary and solid-predominant adenocarcinomas have the highest probability of recurrence.
Discriminating AIS from MIA can be difficult and is probably not clinically important
AIS and MIA are both diagnoses of exclusion, made challenging in part because the exclusionary criteria for each are slightly different (2). While both lesions are by definition 3 cm or less in size, AIS is characterized by pure lepidic growth, which is defined as growth of neoplastic cells along alveolar septa without architectural destruction. MIA is predominantly lepidic, but shows focal invasion measuring 5 mm or less. No stromal, vascular, or pleural invasion is allowed in AIS, whereas only the latter two of these criteria apply to MIA. AIS must not exhibit any papillary or micropapillary pattern or intraalveolar tumor cells, while the only other defining criterion for MIA is the absence of necrosis. Accurate assessment of these parameters demands that lesions in which the diagnosis of AIS or MIA is being considered be entirely histologically examined. Adding to this challenge is the relatively frequent occurrence of sclerosis/stromal collapse of the alveolar framework in AIS that can simulate invasion (Figure 1). Elastic stains may highlight breaks in the alveolar elastic framework in MIA, but interpretation of this sometime subtle finding can be difficult (Figure 2).
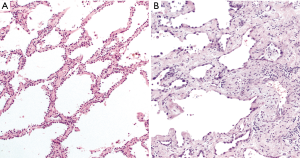
The data on interobserver variability in categorizing AIS from MIA are limited. In one study of 296 surgically resected lung adenocarcinomas reviewed by two pathologists, there was disagreement between AIS and MIA in six cases (2%) (4). Another study found moderate interobserver agreement in the distinction between “typical” cases of non-invasive and invasive lung adenocarcinoma (ĸ =0.55), but poor agreement (ĸ =0.08) for cases participating pathologists considered “problematic” (5). However, discriminating between AIS and MIA may not be clinically important, as multiple validation studies have shown 100% 5-year disease-free survival (DFS) in both tumor types when completely resected (3,6-9). This has led some to propose reassigning completely resected MIA to stage 0 instead of the current stage IA1 designation (9).
Some lepidic-predominant adenocarcinomas behave like AIS and MIA
The reported 5-year DFS for lepidic-predominant lung adenocarcinoma (LPA) is in the range of 70–100% (10-13). A higher proportion of lepidic growth might be reasonably surmised to confer a better prognosis. Recent data have indeed borne this out. In one study, no recurrences were observed in resected lung adenocarcinomas with >50% lepidic growth pattern (12). Another study found that the 5-year observed survival rate of 67% for patients with tumors that are >80% lepidic was significantly better as compared to 56% for patients whose tumors exhibit a lower proportion of lepidic growth (13).
Complex acinar/cribriform/fused glands represent a high-grade pattern
In the current WHO classification, the acinar pattern includes a histologic spectrum ranging from simple acini to more complex acinar arrays/fused glands to cribriform arrangements. Accumulating data indicate that among these, only lung adenocarcinomas in which simple acinar structures predominate have an intermediate risk of recurrence. Not only is the recurrence-free survival (RFS) of complex glandular-predominant adenocarcinomas significantly worse than that of adenocarcinomas with a predominance of simple acini (50 vs. 73 months), it is comparable to the RFS of solid-predominant adenocarcinoma (14).
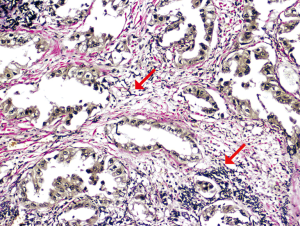
Cribriform growth is likewise an adverse prognostic feature. One study showed the 5-year recurrence-free probability (RFP) of cribriform-predominant lung adenocarcinoma is comparable to that of solid-predominant and micropapillary-predominant adenocarcinoma at 70% and significantly worse than that of simple acini-predominant and papillary-predominant adenocarcinomas, which have a RFP of 87% and 83%, respectively (15). Recognizing even a minor cribriform component appears to be clinically important. As little as 10% cribriform growth in acinar-predominant lung adenocarcinoma confers a significantly increased risk of recurrence (15). Because they have DFS similar to tumors having a predominant pattern recognized to be high grade (e.g. solid or micropapillary), it has been suggested that lung adenocarcinoma with a predominant complex glandular pattern featuring fused or cribriform glands, also be considered high grade (Figure 3) (16).
Papillary-predominant adenocarcinomas are morphologically and prognostically heterogenous
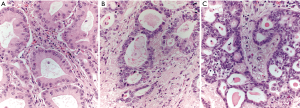
While papillary-predominant lung adenocarcinomas are generally considered intermediate prognosis tumors, not all studies have reached the same conclusion. In one study that reported a 38% 5-year DFS among papillary-predominant lung adenocarcinomas, papillary growth was strictly defined as a very clear-cut papillary architecture with mostly pleomorphic high-grade tumor cells (11). It would appear, however, that not all papillae are created equal with respect to clinical outcome. In a follow-up study, papillary lung adenocarcinomas were placed into three groups based on their architectural and cytologic characteristics: (I) “pseudolepidic” growth with small to medium-sized papillae and slight nuclear atypia, (II) moderate atypia and medium-sized papillae with papillary thyroid carcinoma-like growth, and (III) highly variable-sized papillae with marked atypia (17). Tumors exhibiting highly variable papillae with marked atypia had a significantly worse DFS of 49.9 months as compared to 67.1 months for tumors with “pseudolepidic” growth and slight atypia and 56.8 months for the group with moderate atypia and medium-sized papillae, suggesting that a single category of papillary-predominant lung adenocarcinoma may not be adequate for prognostication (Figure 4).

Discussing the classification of papillary lung adenocarcinoma requires a note of caution. Interpreting a papillary pattern in atelectatic or artifactually compressed lung tissue presents difficulties in that AIS can appear to line central fibrovascular cores, simulating papillary growth (18).
Small amounts of micropapillary pattern affect prognosis
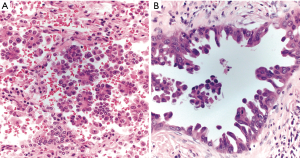
Micropapillary growth in lung adenocarcinoma is associated with a host of adverse prognostic factors, including lymphatic and pleural invasion, nodal metastases and advanced stage disease (19). In one study, the 5-year DFS for micropapillary-predominant lung adenocarcinoma was 0% (19). Even a small amount of micropapillary pattern has a negative clinical impact. Overall survival is significantly worse in tumors with as little as 1% micropapillary pattern (20). Classically, the micropapillary pattern features papillary tufts, florets, and rounded intraalveolar clusters of tumors cells lacking fibrovascular cores. Other forms of micropapillary pattern with similarly unfavorable prognosis have been recognized more recently. The filigree form consists of delicate lacelike narrow stacks of tumor cells piled at least three nuclei high to exclude tangentially cut glands, which lack fibrovascular cores (21). In the stromal form of micropapillary pattern, clusters of tumor cells infiltrate stroma within cleft-like spaces (Figure 5) (22).
Other growth patterns associated with poor prognosis
Solid-predominant lung adenocarcinoma is grouped along with micropapillary-predominant lung adenocarcinoma as a high-grade pattern. It does however exhibit slightly better behavior than micropapillary-predominant lung adenocarcinoma, showing a 5-year DFS in the range of 39–74% in surgically resected cases (6,8). When considering a diagnosis of solid adenocarcinoma, it is important to establish the tumor is in fact an adenocarcinoma and that it is of lung origin by utilizing lung-specific immunohistochemical markers such as TTF-1 and possibly employing histochemical staining to detect mucin.
Most, but by no means all studies, have shown that colloid adenocarcinoma and invasive mucinous adenocarcinoma (IMA) of the lung have a similarly non-favorable prognosis. Both colloid adenocarcinoma and IMA demonstrated a 5-year DFS of 51% in one study of surgically resected cases and in another study, the DFS for colloid adenocarcinoma and IMA was similar at 71% and 75%, respectively (3,6). Contrasting with this are data from a different study in which the DFS of surgically resected IMA of 88 months was better than the 56-month DFS observed for lung adenocarcinoma overall (11).
There have been several reports of pulmonary adenocarcinoma with predominantly intraalveolar single tumor cells mimicking desquamative interstitial pneumonia (23-26). The clinical outcome in these cases has been variable and whether this growth pattern can be considered high grade remains to be determined.
Pattern-drive treatment of lung adenocarcinoma
The National Comprehensive Cancer Network (NCCN) does not recommend treatment beyond complete resection for stage I lung carcinoma, except stage IB tumors with high-risk factors, such as being poorly differentiated, for which adjuvant chemotherapy may be considered (27). Adjuvant chemotherapy has in fact been shown to improve DFS for completely resected stage IB micropapillary-predominant and solid-predominant lung adenocarcinomas, but not acinar-predominant and papillary predominant tumors (6,28). Unfortunately, adjuvant chemotherapy did not to impact overall survival in these cases.
Potential modifications to lung adenocarcinoma classification
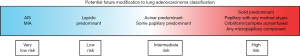
Based on amassed data, it appears likely that the WHO lung adenocarcinoma classification will need to be modified for various patterns to align more accurately with prognosis. Their similar behavior, coupled with the inherent difficulty in separating AIS and MIA, make it attractive to consider collapsing them into one category. While simple acinar formations are associated with an intermediate prognosis when they are the predominant growth pattern, data support recognizing a new category of high-grade tumors with 10% or more complex acinar, cribriform, or fused glands. Categorizing papillary adenocarcinoma with marked atypia as high grade also appears warranted. Lastly, with respect to micropapillary pattern, if it is present in any amount, classifying a tumor a micropapillary lung adenocarcinoma appears indicated (Figure 6). Promoting awareness of different micropapillary appearances in future modifications to the classification scheme will be worthwhile for improving detection of this aggressive histologic pattern.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sanja Dacic) for the series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2019.12.30). The series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” was commissioned by the editorial office without any sponsorship or funding. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. editors. WHO classification of Tumours of Lung Pleura, Thymus and Heart. 4th ed. Lyon: IARC, 2015.
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Boland JM, Froemming AT, Wampfler JA, et al. Adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive pulmonary adenocarcinoma-analysis of interobserver agreement, survival, radiographic characteristics, and gross pathology in 296 nodules. Hum Pathol 2016;51:41-50. [Crossref] [PubMed]
- Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Gu J, Lu C, Guo J, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol 2013;107:474-80. [Crossref] [PubMed]
- Murakami S, Ito H, Tsubokawa N, et al. Prognostic value of the new IASLC/ATS/ERS classification of clinical stage IA lung adenocarcinoma. Lung Cancer 2015;90:199-204. [Crossref] [PubMed]
- Chen T, Luo J, Gu H, et al. Should minimally invasive lung adenocarcinoma be transferred from stage IA1 to stage 0 in future updates of the TNM staging system? J Thorac Dis 2018;10:6247-53. [Crossref] [PubMed]
- Duhig EE, Dettrick A, Godbolt DB, et al. Mitosis trumps T stage and proposed International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification for prognostic value in resected stage 1 lung adenocarcinoma. J Thorac Oncol 2015;10:673-81. [Crossref] [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Strand TE, Rostad H, Strom EH, et al. The percentage of lepidic growth is an independent prognostic factor in invasive adenocarcinoma of the lung. Diagn Pathol 2015;10:94. [Crossref] [PubMed]
- Kuang M, Shen X, Yuan C, et al. Clinical significance of complex glandular patterns in lung adenocarcinoma: clinicopathologic and molecular study in a large series of cases. Am J Clin Pathol 2018;150:65-73. [Crossref] [PubMed]
- Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol 2014;27:690-700. [Crossref] [PubMed]
- Moreira AL, Joubert P, Downey RJ, et al. Cribriform and fused glands are patterns of high-grade pulmonary adenocarcinoma. Hum Pathol 2014;45:213-20. [Crossref] [PubMed]
- Warth A, Muley T, Harms A, et al. Clinical relevance of different papillary growth patterns of pulmonary adenocarcinoma. Am J Surg Pathol 2016;40:818-26. [Crossref] [PubMed]
- Thunnissen E, Belien JA, Kerr KM, et al. In compressed lung tissue microscopic sections of adenocarcinoma in situ may mimic papillary adenocarcinoma. Arch Pathol Lab Med 2013;137:1792-7. [Crossref] [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [Crossref] [PubMed]
- Lee G, Lee HY, Jeong JY, et al. Clinical impact of minimal micropapillary pattern in invasive lung adenocarcinoma: prognostic significance and survival outcomes. Am J Surg Pathol 2015;39:660-6. [Crossref] [PubMed]
- Emoto K, Eguchi T, Tan KS, et al. Expansion of the concept of micropapillary adenocarcinoma to include a newly recognized filigree pattern as well as the classical pattern based on 1468 stage i lung adenocarcinomas. J Thorac Oncol 2019;14:1948-61. [Crossref] [PubMed]
- Ohe M, Yokose T, Sakuma Y, et al. Stromal micropapillary component as a novel unfavorable prognostic factor of lung adenocarcinoma. Diagn Pathol 2012;7:3. [Crossref] [PubMed]
- Miyaoka M, Hatanaka K, Iwazaki M, et al. Pulmonary adenocarcinoma mimicking desquamative interstitial pneumonia: report of 2 cases with genetic analysis. Int J Surg Pathol 2018;26:655-9. [Crossref] [PubMed]
- Hirsch E, Jagirdar J, Nazarullah A. Pulmonary adenocarcinoma, intra-alveolar variant: a rare entity mimicking desquamative interstitial pneumonia. Int J Surg Pathol 2018;26:185-9. [Crossref] [PubMed]
- Raparia K, Ketterer J, Dalurzo ML, et al. Lung tumors masquerading as desquamative interstitial pneumonia (DIP): report of 7 cases and review of the literature. Am J Surg Pathol 2014;38:921-4. [Crossref] [PubMed]
- Mutton AE, Hasleton PS, Curry A, et al. Differentiation of desquamative interstitial pneumonia (DIP) from pulmonary adenocarcinoma by immunocytochemistry. Histopathology 1998;33:129-35. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 5.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Luo J, Huang Q, Wang R, et al. Prognostic and predictive value of the novel classification of lung adenocarcinoma in patients with stage IB. J Cancer Res Clin Oncol 2016;142:2031-40. [Crossref] [PubMed]