
Anti-PDL1 effect in squamous non-small cell lung cancer
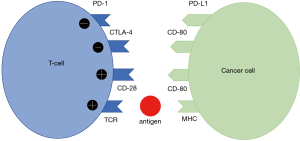
The landmark paper published in 1996 experimentally demonstrated for the first time the idea that one of the immune system checkpoints, Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4), could be targeted with a monoclonal antibody to enhance anti-tumor immunity, which eventually led to treatment of cancer in mouse model (1). This was a discovery that granted the authors the 2018 Nobel prize in Medicine. Fifteen years after the publication of the paper, the first monoclonal antibody targeting human CTLA-4 (ipilimumab), was approved to treat melanoma (2). Other immune checkpoints were subsequently studied as potential therapeutic targets, including programmed death receptor ligand 1 (PD-L1) (3). Figure 1 depicts the various cell receptors position and their effect on T-Cells. Various trials demonstrated the effectiveness of inhibitors of programmed death receptor 1 (PD-1) and its ligand (PD-L1) as treatment options for squamous and non-squamous non-small cell lung cancer (NSCLC) (4-14). A complete list of immune checkpoint inhibitors either FDA approved or still under clinical trials are shown in Table 1. Not all PD-1/L1 inhibitors have the same efficacy in first line therapy for NSCLC, even after combination with chemotherapy. We believe that the reason of the different clinical performance among these agents is related to study design.

Full table
In a recent multicenter randomized double-blinded Phase 3 clinical trial, Paz-Ares et al. (15) reported on 559 patients with untreated metastatic, squamous NSCLC who receive either pembrolizumab (PD-1 inhibitor) plus chemotherapy (N=278) or chemotherapy plus placebo (N=281). A significantly better overall survival and progression-free survival were seen in the pembrolizumab-chemotherapy group compared to the chemotherapy-placebo group, with a HR for death of 0.64 (95% CI, 0.49 to 0.85; P<0.001) and HR for disease progression of disease or death of 0.56 (95% CI, 0.45 to 0.7; P<0.001). The difference in median overall survival and progression free survival between the two arms was 4.6 and 1.6 months respectively, both in favor of pembrolizumab. The benefit was observed in all PD-L1 proportion score subgroups in the progression-free survival and in all subgroups of the overall survival, with the exception of PD-L1 ≥50% that was associated with a trend toward better survival in pembrolizumab group but did not reach statistical significance (HR 0.64, 95% CI, 0.37 to 1.10). Other randomized clinical trials (RCTs) (6,11,14) have investigated the association between PD-L1 tumor proportion score and treatment activity.
Immunotherapy combination improved outcomes compared to placebo combination regardless of the PD-L1 expression score (6,11,14,15). The role of this biomarker is still not completely defined in many studies (6,11,14,15) but recently Paz-Ares and colleagues reported a relationship between higher PD-L1 expression and longer progression-free survival (11). Among our unpublished ongoing meta-analysis on NSCLC, we found HR for progression free survival of 0.63 (95% CI, 0.47 to 0.84) among those with PD-L1 expression of 50% or more favoring pembrolizumab monotherapy over chemotherapy but no difference among those less than 50%.
Another point that warrants discussion is the number of adverse events that occurred during the study period. Adverse events of grade 3 or higher that occurred more frequently in the chemotherapy-pembrolizumab group than in the chemotherapy-placebo group included pneumonitis, reported in the literature as an uncommon but potentially life-threatening complication. Therefore, a recent meta-analysis has investigated the occurrence of pneumonitis as an adverse event in PD-1 and PD-L1 inhibitors. The study reported a higher incidence of pneumonitis with the use of PD-1 inhibitors compared with PD-L1 inhibitors. However, high grade pneumonitis occurrence was statistically insignificant upon comparing immunotherapy to chemotherapy (16).
Finally, a cost-effective analysis should be considered when approaching new treatments. A literature review of the recently published papers analyzing this issue (17-25) did not provide uniform results. The minimum and maximal incremental cost-effectiveness ratio (ICER) were $36,493 and $194,372 respectively per quality-adjusted life-years (QALY) (19,20). Some authors found a cost-effectiveness in all PD-L1 tumor proportion scores (23-25), other only in some subgroups (17,21,22), and others only minimal or no cost-effectiveness (18-20). No definitive conclusion can be therefore drawn, until further analyses are performed.
Paz-Ares and colleagues (15) should be congratulated for their trial which is one of the largest series analyzing pembrolizumab as a treatment option for NSCLC. Other PD1/PD-L1 inhibitors have been investigated, like nivolumab (4,7,10,13) and atezolizumab (9,12), but couldn’t reach a significant survival benefit, as monotherapy or in combination with chemotherapy, for NSCLC as with pembrolizumab.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.02.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734-6. [Crossref] [PubMed]
- FDA Approves YERVOYTM (ipilimumab) for the Treatment of Patients with Newly Diagnosed or Previously-Treated Unresectable or Metastatic Melanoma, the Deadliest Form of Skin Cancer. (accessed January 8, 2020). Available online: https://news.bms.com/press-release/rd-news/fda-approves-yervoy-ipilimumab-treatment-patients-newly-diagnosed-or-previousl
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Govindan R, Szczesna A, Ahn MJ, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3449-57. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Borghaei H, Hellmann MD, Paz-Ares LG, et al. Nivolumab (Nivo) + platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with <1% tumor PD-L1 expression: Results from CheckMate 227. J Clin Oncol 2018;36:9001. [Crossref]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36:LBA9000. [Crossref]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Rahouma M, Baudo M, Yahia M, et al. Pneumonitis as a complication of immune system targeting drugs?—a meta-analysis of anti-PD/PD-L1 immunotherapy randomized clinical trials. J Thorac Dis 2019;11:521-34. [Crossref] [PubMed]
- Loong HH, Wong CKH, Leung LKS, et al. Cost Effectiveness of PD-L1-Based Test-and-Treat Strategy with Pembrolizumab as the First-Line Treatment for Metastatic NSCLC in Hong Kong. Pharmacoecon Open 2019. [Epub ahead of print].
- Liao W, Huang J, Hutton D, et al. Cost-effectiveness analysis of first-line pembrolizumab treatment for PD-L1 positive, non-small cell lung cancer in China. J Med Econ 2019;22:344-9. [Crossref] [PubMed]
- Zhou K, Jiang C, Li Q. Cost-effectiveness analysis of pembrolizumab monotherapy and chemotherapy in the non-small-cell lung cancer with different PD-L1 tumor proportion scores. Lung Cancer 2019;136:98-101. [Crossref] [PubMed]
- Zeng X, Wan X, Peng L, et al. Cost-effectiveness analysis of pembrolizumab plus chemotherapy for previously untreated metastatic non-small cell lung cancer in the USA. BMJ Open 2019;9:e031019. [Crossref] [PubMed]
- She L, Hu H, Liao M, et al. Cost-effectiveness analysis of pembrolizumab versus chemotherapy as first-line treatment in locally advanced or metastatic non-small cell lung cancer with PD-L1 tumor proportion score 1% or greater. Lung Cancer 2019;138:88-94. [Crossref] [PubMed]
- Chouaid C, Bensimon L, Clay E, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer 2019;127:44-52. [Crossref] [PubMed]
- Insinga RP, Vanness DJ, Feliciano JL, et al. Cost-effectiveness of pembrolizumab in combination with chemotherapy versus chemotherapy and pembrolizumab monotherapy in the first-line treatment of squamous non-small-cell lung cancer in the US. Curr Med Res Opin 2019;35:1241-56. [Crossref] [PubMed]
- Huang M, Lopes G de L, Insinga RP, et al. Cost-effectiveness of pembrolizumab versus chemotherapy as first-line treatment in PD-L1-positive advanced non-small-cell lung cancer in the USA. Immunotherapy 2019;11:1463-78. [Crossref] [PubMed]
- Weng X, Luo S, Lin S, et al. Cost-utility analysis of pembrolizumab versus chemotherapy as first-line treatment for metastatic non-small cell lung cancer with different PD-L1 expression levels. Oncol Res 2020;28:117-25. [Crossref] [PubMed]


