Anti PD-1 monoclonal antibody induced autoimmune diabetes mellitus: a case report and brief review
Introduction
Immune checkpoint inhibitors, such as programmed cell death 1 (PD-1) blockades and cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) blockades, have shown promising anti-tumor effects. And anti PD-1 antibodies have been applied on more and more cancer patients. Except for their impressive anti-tumor effects, some unique adverse effects called immune-related adverse effects (irAEs), such as rash, diarrhea, colitis, hepatitis, endocrinopathy and pneumonitis, have been described as common irAEs (1,2).
Anti PD-1 monoclonal antibody is a humanized IgG4 anti PD-1 antibody. It can block PD-1/PD-L1 pathway, which has a negative impact on T cells function, to enhance the anti-tumor effects of hosts’ T cells (3). Nowadays, anti PD-1 monoclonal antibody has been evaluated in clinical trials for many types of cancers. As an immune checkpoint inhibitor, anti PD-1 monoclonal antibody can also cause irAEs including endocrinopathy. Autoimmune diabetes mellitus was a kind of endocrinopathy that can be induced by anti PD-1 therapy but not a very common side effect with potential fatality during therapy. In this report, we described an autoimmune diabetes mellitus induced by anti PD-1 monoclonal antibody in non-small cell lung cancer (NSCLC) treatment. And we presented the following case in accordance with the CARE guideline (4).
Case presentation
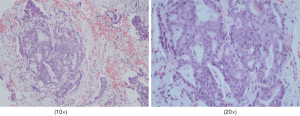
Our patient, a 73-year-old male with no diabetes mellitus history, was diagnosed as NSCLC in May 2nd 2017. Gene examination found that epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS, KRAS and KRAF were wide type. He used pemetrexed and cisplatin (pemetrexed 500 mg/m2 d1; cisplatin 75 mg/m2 d1–3; Q21d) as first-line therapy for 3 cycles. Due to renal dysfunction after the third cycle, the therapy was changed to pemetrexed and oxaliplatin (pemetrexed 500 mg/m2 d1; oxaliplatin 130 mg/m2 d1; Q21d). After one cycle the oxaliplatin was replaced by carboplatin (pemetrexed 500 mg/m2 d1; carboplatin AUC=5 d1; Q21d) because of the hands paresthesia. In October 9th 2017, patient’s chest computed tomography (CT) suggested a progression of disease. Thus, he started to use paclitaxel liposome, carboplatin and bevacizumab as a regimen (paclitaxel liposome 175 mg/m2 d1; carboplatin AUC=5 d1; bevacizumab 7.5 mg/kg d1; Q21d). After 7 cycles treatment in May 3rd 2018, CT scan showed a progression disease. Before the anti PD-1 therapy the clinical classification of his cancer was III B (T2bN3M0) and we did a pathological examination (Figure 1). And the chest CT scan showed a 5.0 cm × 4.0 cm mass in right upper lobe (Figure 2A). The abdominal and pelvic CT scan showed no sign of metastasis (Figure 2B,C) and brain MRI was also normal. His blood glucose was normal at this time. The immune checkpoint inhibition therapy was applied then. He started to receive anti PD-1 monoclonal antibody 200 mg every 3 weeks. And this therapy did get his disease controlled (Figure 2D). His blood glucose level was normal at this time.
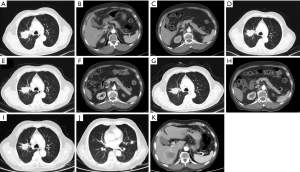
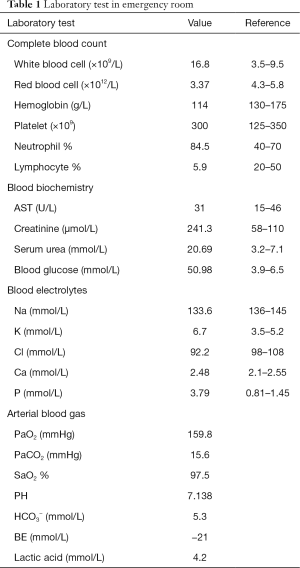
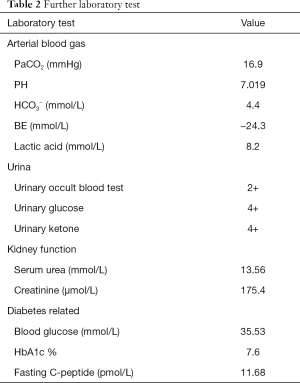
Before the tenth cycle of this anti PD-1 monoclonal antibody treatment, his blood glucose was 4.4 mmol/L and chest CT scan showed a stable disease and abdominal CT scan did not show pancreatic metastasis (Figure 2E,F). On Dec 10th, 2018, 12 days after the tenth fusion of anti PD-1 monoclonal antibody, the patient was admitted to the emergency room due to vomit, dizzy and tachypnea. There was no polyuria, polydipsia, chest pain, cough, hemoptysis, palpitation, paralysis of limbs and other symptoms. The patient’s temperature was 36.0 °C, pulse was 104 bpm, frequency of the respiratory was 17 per minute and blood pressure was 129/54 mmHg. The laboratory test (Table 1) showed blood glucose was 50.98 mmol/L, arterial blood PH was 7.138, PaCO2 was 15.6 mmHg, bicarbonate was 5.3 mmol/L and lactic acid was 4.2 mmol/L. Considering his hyperglycemia and low arterial blood PH, diabetic ketoacidosis was diagnosed. Intravenous fluid infusion, continuous insulin infusion and anti-infection therapy were applied to him. On Dec 17th 2018, he was transferred to ICU for further treatment. ICU laboratory test (Table 2) showed that his blood glucose was 35.53 mmol/L, arterial blood PH was 7.019, bicarbonate was 4.4 mmol/L, lactic acid was 8.2 mmol/L, and urinary ketone was 4+. Further examination showed fasting C-peptide was 11.68 pmol/L which indicated an insufficient function of pancreas islet beta cells. However, relative auto-antibodies were negative. After intravenous insulin fusion, hemofiltration and other treatment, the DKA was cured. Then patient was managed with insulin pump for short term blood glucose control. Subsequently it was switched as glargine and aspart subcutaneous injection for long term treatment.
Full table
Full table
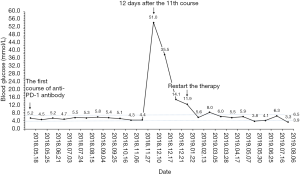
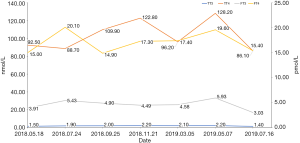
Because of the adverse effect, his 11th cycle of anti PD-1 monoclonal antibody treatment was delayed about 1 month. The chest and abdominal CT scan did not find a progressed disease or any abnormalities in his pancreas (Figure 2G,H). It was concerned that if the patients had anti PD-1 regimen induced autoimmune diabetes and had episode of DKA whether the therapy would still be safe for the patients. It was a discrepancy in making the decision in clinical practice. Considering anti PD-1 monoclonal antibody could control his cancer well and his blood glucose was stable with subcutaneous insulin injection, we decided to restart the regimen. Encouragingly, the following laboratory test showed that his blood glucose was controlled well with the long-acting insulin and prandial short-acting insulin during the restarted anti PD-1 therapy. And other endocrine adverse effects such as abnormal thyroid function did not occur. The lung cancer was also stable until Jun 25th, 2019. Although, before his 18th cycle of this anti PD-1 monoclonal antibody, the primary lesion in right upper lobe was still stable (Figure 2I). Unfortunately, CT scan showed a 1.4 cm × 1.1 cm nodule in left lingular segment (Figure 2J) and a 5.0 cm × 3.8 cm mass in right liver lobe (Figure 2K). Thus it was considered as a progressed disease. Considering that the primary lesion was still stable and the patient said “This therapy has controlled my disease for a long time and improved my life quality. With the help of this agent I can have a better life and longer time to stay with my family. I would like to continue this therapy for further treatment.” After discussion, since continuing this anti PD-1 monoclonal antibody therapy would still make him get benefit, the regimen was applied to date. His blood glucose level during the whole anti PD-1 therapy was shown in Figure 3. And here we summarized his laboratory test of thyroid function in Figure 4 and his whole treatment in Figure 5.
Discussion
Cancer can suppress immune responses through several negative regulatory pathways, such as PD-1/PD-L1 pathway. PD-1 will express on activating T cells. Once it binds to its two ligands, PD-L1 and PD-L2, it will cause a negative impact and attenuate the activity of T cells. Thus, blocking this axis can enhance T cells anti-tumor activity and function (5,6). Nivolumab and pembrolizumab are two humanized IgG4 antibodies that block the PD-1. These antibodies have shown their promising effects in NSCLC treatment (7-9) and have been approved by FDA for metastatic NSCLC treatment (1). There are lots of other anti PD-1 monoclonal antibodies which can block PD-1/PD-L1 mediated negative signal (10).
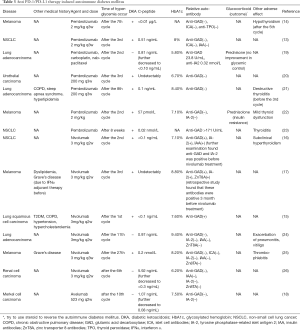
With the negative regulatory function, PD-1/PD-L1 pathway is not only involved in assisting cancer cells to escape eradication by immune system, but also plays an indispensable role in maintain immune homeostasis (11). Considering that, PD-1 inhibit therapy will break its function in immune homeostasis at the same time which may lead autoimmune reaction and cause irAEs subsequently. Endocrine system is also affected commonly. A study found that the proportion of pembrolizumab related endocrine adverse effect was 6.9% (12). And with gradually extensive use of nivolumab and pembrolizumab, several autoimmune diabetes mellitus induced by these two agents have been reported in clinical practice (13-17). Anti-PD-L1 agent, avelumab, also could induce rapid type 1 diabetes mellitus (18). We had summarized these reports in the Table 3. Some of them suffered thyroid dysfunction or other endocrinopathy simultaneously (14,17). HbA1c was increased in most cases which indicated that patients had suffered hyperglycemia for some time. However, a patient showed a slightly lower HbA1c after pembrolizumab treatment with a significantly increased blood glucose level (19). Considering that he got chemotherapy at the same time which might affect his hemoglobin level and his blood glucose elevated in a short time, HbA1c might not accurate enough to represent the average level of blood glucose. C-peptide is an indicator to reflect the function of islet β cells. Most patients showed a decrease C-peptide level which indicated β cells dysfunction. Our patient also showed an obviously decreased C-peptide level in both fasting and stimulating state. There was also a hyperglycemic patient with a normal C-peptide level at first. But his serum C-peptide level gradually decreased to <0.3 ng/dL in 8 days (26). This indicated that when hyperglycemic with normal C-peptide level occurred in patients with the anti PD-1/PD-L1 treatment, treatment induced diabetes should also be considered and monitored the C-peptide level was necessary. In conventional autoimmune diabetes, autoimmune antibodies would be found elevate. In some anti PD-1 therapy induced autoimmune diabetes, researchers found GADA, IA-2, IAA and ZnT8A turned to positive or increased obviously (15-17,19,23). And the presence/absence of GADA showed the relevance with the onset of autoimmune diabetes mellitus during anti PD-1/PD-L1 therapy (17,27). But there were also some cases showed patients with normal level of relative autoimmune antibodies, which suggested that the genesis of anti PD-1/PD-L1 therapy induced autoimmune diabetes was not exactly same as conventional autoimmune diabetes (13,14,18,20-22,24-26). Relative autoimmune antibodies were also negative in this patient. As glucocorticoids could be applied to treat irAEs and high dose of corticosteroids could prevent long-term hormone deficiency had been described (1), some clinicians tried to use glucocorticoids to rescue the function of islet. But this treatment could not reverse the dysfunction of islet and could cause insulin resistance in some patients inversely (19,22). Furthermore, it was reported a patient used prednisolone for thrombophlebitis, but she still developed diabetes as irAEs, which might mean that glucocorticoids could not prevent the autoimmune diabetes caused by anti PD-1 therapy (25). Patients would need permanent insulin injection to get glycemic control due to unrecoverable islet function. But after appropriate treatment and control with insulin, some patients resume former therapy safely (14,17). Some anti PD-1 antibodies are engineered to bind to FcγRI insufficiently to enhance the anti-tumor effect of the agent. This strategy may further reduce autoreactive immune cells elimination. And it needs more investigation to evaluate whether the frequency of autoimmune diabetes mellitus in using these antibodies is higher than nivolumab and pembrolizumab. In our case, glargine and aspart were used for our patient to control his blood glucose level. And his glycemic control was good even with the further anti PD-1 therapy.
Full table
Anti PD-1/PD-L1 therapy has also been used in patients with T2DM history (15,28). One patient occurred DKA just after the first course of therapy with low C-peptide and positive GADA (15). Although another patient did show the elevated blood glucose, which was considered as a result of his thyroid storm instead of destruction of islet function. And his C-peptide level was still in normal range (28). Thus, it may be important to investigate whether T2DM patients will be more sensitive to this kind of irAE.
There has been some research to investigate the role of PD-1/PD-L1 in autoimmune diabetes mellitus. Macrophages, usually located near blood vessels in pancreas islets, had been found have ability to capture particles which contained insulin and present auto-antigens to relative T cells (29). A study had confirmed that pancreas islets resident macrophages had an important role in initiation of diabetes in nonobese diabetic (NOD) mice. It had been found that using anti-CSF-1R antibody to delete resident macrophages could reduce diabetes incidence in NOD mice. Moreover, this protective effect could be vanished by anti PD-1 antibodies (30). PD-1 was found to express on auto-reactive T cells (31) and PD-L1 was found to express on pancreas islet cells (32). Yet it had been reported that expression of PD-L1 on parenchymal cells could prevent the presence of autoimmune diabetes (32). Rajasalu et al. also reported that lack of PD-L1 on target cells and PD-1 on T cells was related to preproinsulin specific CD8+ T cells induced autoimmune diabetes mellitus (33). A study in type 1 diabetes mellitus patients was also found that defective expression of PD-1 might cause a negative effect on regular T cells (Treg) (34). Another research in NOD mice treated with PD-1/PD-L1 blockade showed no relation between insulin autoantibody and presence of diabetes. But it was found that the auto-reactive T cells expanded after PD-1/PD-L1 blockage (31). Even here exist these researches, but the pathogenesis of anti PD-1 agent induced autoimmune diabetes mellitus still need to be further investigated.
In this report, we showed a case of anti PD-1 monoclonal antibody induced autoimmune diabetes mellitus and DKA in NSCLC treatment. This adverse effect can be life-threatening but its most symptoms are nonspecific that may not get patients attention. Since that, it is necessary to inform patients the potential risk of autoimmune diabetes mellitus when they are in anti PD-1 monoclonal antibody treatment and how to identify the symptoms of hyperglycemia and DKA in order to get medical care timely. At least blood glucose and HbA1c should be examined before and during the anti PD-1 monoclonal antibody treatment. C-peptide level and autoimmune antibodies should also be considered into detection. One of limitation in our case was that we did not detect the relative autoimmune antibodies and serum C-peptide level dynamically after patient recovering from DKA. Thus, we could not identify whether autoimmune antibodies would become positive latter or not and could not evaluate the function of the islet directly. Because most endocrinopathies associated with anti PD-1/PD-L1 therapy have no specific symptoms, it should suggest patients diagnosed with autoimmune diabetes mellitus to have other hormone test to exclude other endocrinopathies. And according to our experience in this case, patients with anti PD-1 monoclonal antibody induced diabetes mellitus could continue the therapy with detection of blood glucose and insulin replacement. But our experience should not be used to represent all patients. Thus, it still requires large and long-term research to further evaluate anti PD-1 monoclonal antibody induced autoimmune diabetes mellitus.
Acknowledgments
Funding: This study was supported in part by a grant from National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036) and a grant from Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131), National key research & development project (2016YFC0902300), Major disease clinical skills enhancement program of three year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A), “Dream Tutor” Outstanding Young Talents Program (fkyq1901), key disciplines of Shanghai Pulmonary Hospital (2017ZZ02012), grant of Shanghai Science and Technology Commission (16JC1405900).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.05). CZ serves as an unpaid Editor-in-Chief of Translational Lung Cancer Research. YH serves as the unpaid editorial board member of Translational Lung Cancer Research from Jan 2020 to Dec 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was also obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol 2016;2:1346-53. [Crossref] [PubMed]
- Shohdy KS, Abdel-Rahman O. Neurological complications of anti-PD-1 antibodies: shall we be more concerned? Transl Cancer Res 2018;7:S436-S438. [Crossref]
- Qin S, Finn RS, Kudo M, et al. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol 2019;15:1811-22. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [Crossref] [PubMed]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Afzal MZ, Dragnev KH, Shirai K. An extended overall survival analysis of pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced non-squamous non-small cell lung cancer. Ann Transl Med 2019;7:S53. [Crossref] [PubMed]
- Salati M, Baldessari C, Cerbelli B, et al. Nivolumab in pretreated non-small cell lung cancer: continuing the immunolution. Transl Lung Cancer Res 2018;7:S91-4. [Crossref] [PubMed]
- Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcγRI has a profound impact on its biological functions. Cancer Immunol Immunother 2018;67:1079-90. [Crossref] [PubMed]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219-42. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Li S, Zhang Y, Sun Z, et al. Anti-PD-1 pembrolizumab induced autoimmune diabetes in Chinese patient: A case report. Medicine (Baltimore) 2018;97:e12907. [Crossref] [PubMed]
- Hakami OA, Ioana J, Ahmad S, et al. A case of pembrolizumab-induced severe DKA and hypothyroidism in a patient with metastatic melanoma. Endocrinol Diabetes Metab Case Rep 2019;2019. [Crossref] [PubMed]
- Lee S, Morgan A, Shah S, et al. Rapid-onset diabetic ketoacidosis secondary to nivolumab therapy. Endocrinol Diabetes Metab Case Rep 2018;2018. [Crossref] [PubMed]
- Godwin JL, Jaggi S, Sirisena I, et al. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer 2017;5:40. [Crossref] [PubMed]
- Gauci ML, Laly P, Vidal-Trecan T, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother 2017;66:1399-410. [Crossref] [PubMed]
- Shibayama Y, Kameda H, Ota S, et al. Case of fulminant type 1 diabetes induced by the anti-programmed death-ligand 1 antibody, avelumab. J Diabetes Investig 2019;10:1385-7. [Crossref] [PubMed]
- Chae YK, Chiec L, Mohindra N, et al. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol Immunother 2017;66:25-32. [Crossref] [PubMed]
- Tohi Y, Fujimoto K, Suzuki R, et al. Fulminant type 1 diabetes mellitus induced by pembrolizumab in a patient with urothelial carcinoma: A case report. Urol Case Rep 2019;24:100849. [Crossref] [PubMed]
- Edahiro R, Ishijima M, Kurebe H, et al. Continued administration of pembrolizumab for adenocarcinoma of the lung after the onset of fulminant type 1 diabetes mellitus as an immune-related adverse effect: A case report. Thorac Cancer 2019;10:1276-9. [Crossref] [PubMed]
- Aleksova J, Lau PK, Soldatos G, et al. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep 2016;2016. [Crossref] [PubMed]
- de Filette JMK, Pen JJ, Decoster L, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol 2019;181:363-74. [Crossref] [PubMed]
- Kumagai R, Muramatsu A, Nakajima R, et al. Acute-onset type 1 diabetes mellitus caused by nivolumab in a patient with advanced pulmonary adenocarcinoma. J Diabetes Investig 2017;8:798-9. [Crossref] [PubMed]
- Sakaguchi C, Ashida K, Yano S, et al. A case of nivolumab-induced acute-onset type 1 diabetes mellitus in melanoma. Curr Oncol 2019;26:e115-8. [Crossref] [PubMed]
- Yamamoto N, Tsurutani Y, Katsuragawa S, et al. A patient with Nivolumab-related Fulminant Type 1 Diabetes Mellitus whose Serum C-peptide Level Was Preserved at the Initial Detection of Hyperglycemia. Intern Med 2019;58:2825-30. [Crossref] [PubMed]
- Usui Y, Udagawa H, Matsumoto S, et al. Association of Serum Anti-GAD Antibody and HLA Haplotypes with Type 1 Diabetes Mellitus Triggered by Nivolumab in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:e41-3. [Crossref] [PubMed]
- Yonezaki K, Kobayashi T, Imachi H, et al. Combination therapy of ipilimumab and nivolumab induced thyroid storm in a patient with Hashimoto's disease and diabetes mellitus: a case report. J Med Case Rep 2018;12:171. [Crossref] [PubMed]
- Vomund AN, Zinselmeyer BH, Hughes J, et al. Beta cells transfer vesicles containing insulin to phagocytes for presentation to T cells. Proc Natl Acad Sci U S A 2015;112:E5496-502. [Crossref] [PubMed]
- Carrero JA, McCarthy DP, Ferris ST, et al. Resident macrophages of pancreatic islets have a seminal role in the initiation of autoimmune diabetes of NOD mice. Proc Natl Acad Sci U S A 2017;114:E10418-27. [Crossref] [PubMed]
- Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;198:63-9. [Crossref] [PubMed]
- Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883-95. [Crossref] [PubMed]
- Rajasalu T, Brosi H, Schuster C, et al. Deficiency in B7-H1 (PD-L1)/PD-1 coinhibition triggers pancreatic beta-cell destruction by insulin-specific, murine CD8 T-cells. Diabetes 2010;59:1966-73. [Crossref] [PubMed]
- Perri V, Russo B, Crino A, et al. Expression of PD-1 Molecule on Regulatory T Lymphocytes in Patients with Insulin-Dependent Diabetes Mellitus. Int J Mol Sci 2015;16:22584-605. [Crossref] [PubMed]