
Implementation of durvalumab maintenance treatment after concurrent chemoradiotherapy in inoperable stage III non-small cell lung cancer (NSCLC)—a German radiation oncology survey
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide (1-3). The majority of these patients are diagnosed with advanced non-small cell lung cancer (NSCLC) (stage III to IV) and multimodal approaches including immune checkpoint inhibition are currently under investigation in various settings (4,5). Administering platinum-based chemotherapy sequentially and/or concurrently to thoracic irradiation resulted in moderate improvements of local control, metastasis-free and overall survival (2,3,6). Dose-escalation in concurrent chemoradiotherapy (cCRT) as well as combination with targeted therapy concurrently and as consolidation treatment provided no benefit in overall survival in inoperable stage III NSCLC (7). In the phase III PACIFIC trial, durvalumab as maintenance treatment following the completion of platinum-based cCRT significantly improved progression-free and overall survival in patients with unresectable stage III NSCLC (8,9). Therefore, maintenance treatment with PD-L1 inhibition after cCRT has become the new standard of care.
However, not all patients are eligible for PD-L1 inhibition after cCRT (10,11) and new evidence-based treatment recommendations require time for implementation in nationwide settings and close interaction of different specialities (12).
In this nationwide survey, we questioned the distribution and clinical settings of durvalumab treatment after cCRT in inoperable stage III NSCLC with critical analysis of treatment breakdowns, observed side effects and summarize follow-up management. Herein, we present the analysis of responses related to durvalumab recommendations.
Methods
The study was approved by the Board of the German Society of Radiation Oncology (DEGRO e.V.) which provided a database of all their members which agreed their willingness to participate. The web-based survey was developed with the open access software LimeSurvey software licensed by the University of Munich and contained 18 potential questions regarding respondent demographics, clinical setting of durvalumab treatment, observed side effects and follow up management. Branching logic was used to tailor the questions on the basis of previous responses, so not all respondents saw the 18 questions.
The data sample was collected through an online anonymized survey of radiation oncologists in Germany. The survey was initially sent to 1,200 potential participants who are all members of the German Society of Radiation Oncology (DEGRO e.V.) on May 31, 2019. A reminder e-mail was sent to all remaining participants on June 24, 2019, to maximize response rate. Participants who had requested to be removed on account of non-applicability were not sent a reminder e-mail.
Results
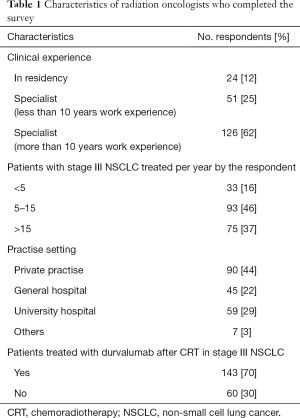
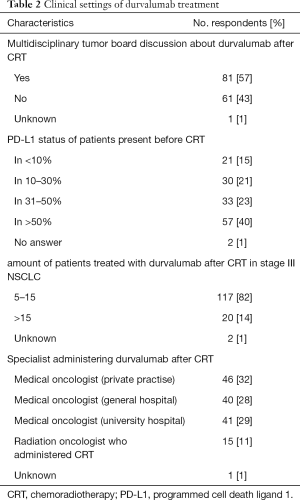
The survey was sent to 1,200 potential respondents who are all members of the German Society of Radiation Oncology (DEGRO e.V.). We received 73 failed or ineligible responses and a total of 255 responses of which 203 (response rate: 18%) were completed and returned and thus eligible for further evaluation. The respondents work in 87 different cities and 44% in a private medical practice, 29% in university and 22% in a general hospital (see Table 1). More than 60% of the respondents had been practicing for more than 10 years after completing residency training. Thirty-seven percent of all respondents treat more than 15 patients with inoperable stage III NSCLC with cCRT at their departments and at least 83% of all respondents five or more patients with stage III NSCLC per annum. 143 (70%) respondents implemented and treated patients with durvalumab after cCRT at their centers. Major reasons for failed implementation in clinical practice reported by the respondents were patient’s ineligibility (42%), lack of required PD-L1 status (25%), decision of medical oncologists (7%) or absence of updated German guidelines (S3-guidelines) (7%) regarding this treatment approach. Respondents working at a university hospitals show higher rates of durvalumab treatment implementation compared to respondents working in medical practice or general hospitals (93% versus 61%, P<0.001). One hundred and seventeen (82%) respondents treated 1–15 patients with cCRT followed by durvalumab and 20 (14%) respondents over 15 patients (see Table 2). The majority (57%) of respondents discuss durvalumab maintenance treatment at initial diagnosis in multidisciplinary tumor boards.
Full table
Full table
PD-L1 status of patients before cCRT start is known in more than 50% of all patients by 40% of all respondents. According to 36% of all respondents, initial tumor cell PD-L1 expression based on IHC assay was present in ≤30% of all patients (see Table 2). Respondents from university hospitals report more frequently availability of PD-L1 status before cCRT compared to others (Mann-Whitney-U-test: z=−2,415, P=0.016).
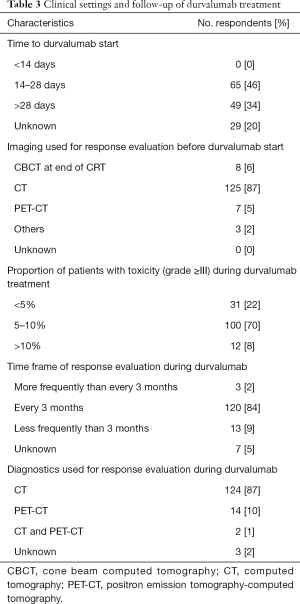
Durvalumab is applied by a medical oncologist in private practice according to 46 (32%) respondents, medical oncologist in a general hospital, medical oncologist at a university hospital and the radiation oncologist who administered cCRT according to 40 (28%), 41 (29%) and 15 (11%) respondents, respectively (see Table 2). No respondent starts durvalumab less than 14 days after cCRT. Sixty-four percent of all (64%) respondents apply durvalumab 14–28 days after cCRT. Thirty-four percent of all respondents start consolidation treatment with durvalumab later than 28 days after cCRT.
Response evaluation before durvalumab start is performed by CT versus other imaging modalities according to 125 (87%) and 18 (13%) of all respondents, respectively.
Severe toxicity requiring hospital admission (≥3 CTCAE) was reported in less than 5% of all patients by 31 (22%) respondents, 5–10% by 100 (70%) respondents and >10% of all patients by 12 (8%) respondents (see Table 3). Twenty (14%) respondents reported pneumonitis (≥3 CTCAE) during durvalumab. Durvalumab treatment discontinuation was reported in more than 20% of all patients by 5 (4%) respondents and in 10–20% of all patients by 27 (19%) respondents.
Full table
Response evaluation during durvalumab treatment is performed every 3 months by 120 (84%) respondents (see Table 3). The majority (87%) of respondents evaluate tumor response with CT and additionally 14 (10%) respondents use PET-CT as a diagnostic modality in the follow-up.
Discussion
As a result of the phase III PACIFIC trial, consolidation treatment with the PD-L1 inhibitor durvalumab after completion of platinum-based chemoradiotherapy has become standard of care with marked improvement of progression-free and overall survival (4,8). Despite the excellent results of this trial, new treatment recommendations need time to be implemented into clinical practice and require the close interaction of different medical specialities such as radiation and medical oncologists.
In this nationwide study, we first surveyed the distribution and clinical settings of durvalumab treatment after cCRT in inoperable stage III NSCLC with focus on treatment obstacles, observed side effects of this treatment and summarize follow-up management.
Seventy percent of all respondents have already implemented and treated patients with durvalumab after cCRT at their cancer centers. This finding goes along with the assumption that clinical implementation requires time to be fulfilled in a nationwide setting. However, approval of durvalumab maintenance treatment in Germany was granted in October 2018 and an implementation rate of 70% reported by our nationwide radiation oncology survey indicates a high and rapid distribution based on the tight timeframe. Importantly, major reasons for failed implementation in clinical practice described by the participants were patient’s ineligibility, decision of medical oncologists or lack of updated recommendations for immune checkpoint inhibition in this setting in the German S3-guidelines. The majority (57%) of the respondents discuss durvalumab maintenance treatment before start of multimodal therapy in tumor board.
Approval for durvalumab maintenance treatment after cCRT in Europe is defined on initial tumor cell PD-L1 expression based on IHC assay. However, PD-L1 status at initial diagnosis was no planned stratification factor in the PACIFIC trial and the exploratory post hoc analysis of OS and PFS in patients with different levels of initial PD-L1 expression, found that, of the 63% of patients with evaluable PD-L1 measurements, patients with PD-L1-negative status might not have benefited from durvalumab. Thus in accordance with the decision by the European Medical Agency (EMA), health insurance does not cover consolidation durvalumab in PD-L1 negative patients in Europe. Our nationwide radiation oncology survey reported low testing rates of PD-L1 at initial diagnosis and this finding should be considered a major barrier to universal adoption and integration in the clinical work-flow especially in countries with durvalumab approval restricted to PD-L1 positive patients. 25% of all respondents who failed to implement durvalumab consolidation treatment reported a lack of required PD-L1 status.
Importantly, there is no proper definition of ineligibility concerning durvalumab maintenance after cCRT. The role of comorbidities i.e., lung and heart function test results before and after cCRT, tumor-related parameters i.e. treated tumor volume, volume of treated lymph node compartment and basic parameters of the radiation treatment planning such as MLD, MHD, V20 and V30 of total lung and heart need an independent comprehensive evaluation to characterize patients ineligibility as well as to predict a treatment-related severe toxicity i.e., grade III pneumonitis (10,11).
Interestingly, no participant started durvalumab in less than 14 days after cCRT. Forty-six percent respondents apply durvalumab treatment 14–28 days after cCRT and even 34% later than 28 days after cCRT. A secondary exploratory post-hoc analysis of PACIFIC revealed a clear benefit for patients starting durvalumab in less than 14 days after completion of cCRT (8,9). Based on this evidence, the current clinical practise is rather different and new studies need to evaluate causes of treatment delay.
Restaging before treatment start is performed using CT thorax and upper abdomen with i.v. contrast by the majority (87%) of all respondents. Our nationwide radiation oncology survey revealed that 6% of all respondents evaluate treatment response before durvalumab start with the last fraction cone-beam CT. Therefore, this diagnostic modality needs to be considered with caution due to less information about treatment response and treatment-related acute side effects.
According to the results of the phase III PACIFIC trial, durvalumab is well tolerated with low grade ≥ III toxicity (8,9) which goes along with the reported findings in our survey. However, data collection about toxicity using our survey bears risks of under- or overrepresentation and should be treated with caution.
Moreover, all grades of radiation pneumonitis were reported to be 20.2% by Antonia et al. and grade 3 or 4 pneumonitis and radiation induced pneumonitis assessed by the investigators were found by to be extremely low (9). In previously studies, symptomatic pneumonitis (grade ≥ III) after cCRT is observed more frequently in 5–16% of all patients (13,14). In addition, differentiation between radiation and/or immune-related pneumonitis in real-world setting is not possible. According to our experience, radiation and immune-mediated pneumonitis may be reported together. Therefore, the reported rate of pneumonitis (grade ≥ III) in our nation-wide radiation oncology survey are in accordance to historical results (13,14). We reported several cases of pneumonitis (grade III) after thoracic radiotherapy and during PD-1 inhibition with nivolumab resulting in an interruption of immune checkpoint inhibition (15).
In our nationwide radiation oncology survey, discontinuation of durvalumab was reported to be low by the respondents. However, several limitations need to be taken into account such as differences in follow-up between radiation oncology departments and private practices, limited follow-up time and inclusion of residents (12%) which may rotate during their residency and may not be in a position to answer questions about surveillance and toxicity.
The number of patients with PD-L1 maintenance treatment after cCRT in inoperable stage III NSCLC as well as number of administering cancer centers will continuously increase. As a result, we are convinced that discontinuation rates of durvalumab treatment will increase as well. Multi-centre prospective trials will be necessary to improve patient monitoring and characterize treatment discontinuation.
The limitation of our study is the low response rate with a sample size of 203 completed responses. Data collection including toxicity data has to be questioned critically and bears risks of bias and under- or overrepresentation. Therefore, our findings have to be interpreted with caution, as they may not be representative of other radiation oncologists who chose not to participate in the survey.
However, the study represents the first survey reviewing clinical settings of durvalumab treatment after cCRT in inoperable stage III NSCLC, revealing several shortcomings, observed side effects and summarize follow-up management.
Conclusions
This nationwide radiation oncologist survey shows the rapid implementation of durvalumab in the multimodal treatment of inoperable stage III NSCLC which is administered by the majority of participants. Major reasons for failed implementation in clinical practice reported by the respondents were patient’s ineligibility, lack of required PD-L1 status, decision of medical oncologists or lack of updated German guidelines (S3-guidelines) regarding this treatment approach. Low testing rates of PD-L1 at initial diagnosis were observed and should be considered a major barrier to universal adoption and integration in the clinical work-flow. No respondent applies durvalumab in less than 14 days after cCRT and reasons of treatment delay need to be evaluated in further studies. Treatment-side effects are in accordance to historical results and need to be considered during and after multimodal therapy and require close clinical monitoring.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.25). Prof. Claus Belka serves in the advisory board of Astrazeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The analysis is in compliance with the principles of the Declaration of Helsinki and its subsequent amendments. The study was approved by the Board of the German Society of Radiation Oncology (DEGRO e.V.) which provided a database of all their members which agreed their willingness to participate.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Postmus PE, Kerr K, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-21. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]
- Shafique MR, Robinson LA, Antonia S. Durvalumab: a potential maintenance therapy in surgery-ineligible non-small-cell lung cancer. Cancer Manag Res 2018;10:931. [Crossref] [PubMed]
- Käsmann L, Eze C, Dantes M, et al. State of clinical research of radiotherapy/chemoradiotherapy and immune checkpoint inhibitor therapy combinations in solid tumours—a German radiation oncology survey. Eur J Cancer 2019;108:50-4. [Crossref] [PubMed]
- Käsmann L, Niyazi M, Blanck O, et al. Predictive and prognostic value of tumor volume and its changes during radical radiotherapy of stage III non-small cell lung cancer. Strahlenther Onkol 2018;194:79-90. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Hosoya K, Fujimoto D, Kawachi H, et al. Ineligibility for the PACIFIC trial in unresectable stage III non-small cell lung cancer patients. Cancer Chemother Pharmacol 2019;84:275-80. [Crossref] [PubMed]
- Sakaguchi T, Ito K, Furuhashi K, et al. Patients with unresectable stage III non-small cell lung cancer eligible to receive consolidation therapy with durvalumab in clinical practice based on PACIFIC study criteria. Respir Investig 2019;57:466-71. [Crossref] [PubMed]
- Björk Brämberg E, Jensen I, Kwak L. Nationwide implementation of a national policy for evidence-based rehabilitation with focus on facilitating return to work: a survey of perceived use, facilitators, and barriers. Disabil Rehabil 2020;42:219-27. [Crossref] [PubMed]
- Xu Y, Ma S, Ji Y, et al. Concomitant chemoradiotherapy using pemetrexed and carboplatin for unresectable stage III non-small cell lung cancer (NSCLC): preliminary results of a phase II study. Lung Cancer 2011;72:327-32. [Crossref] [PubMed]
- Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: A randomized phase II locally advanced multi-modality protocol. J Clin Oncol 2005;23:5883-91. [Crossref] [PubMed]
- Manapov F, Roengvoraphoj O, Dantes M, et al. Pneumonitis in irradiated lungs after nivolumab: a brief communication and review of the literature. J Immunother 2018;41:96-9. [Crossref] [PubMed]


