Feasibility and safety of PD-1/L1 inhibitors for non-small cell lung cancer in front-line treatment: a Bayesian network meta-analysis
Introduction
Non-small cell lung cancer (NSCLC) occupies 85% of all lung cancer cases (1). The majority of NSCLC patients are diagnosed at advanced stage and the prognosis for these patients is poor, therefore systematic therapy is the primary choice. Nearly 30–40% NSCLC patients owned sensitive mutations, that may suitable for corresponding tyrosine kinase inhibitors. However, effective treatments for other patients without mutations are rather limited. The response rate of traditional chemotherapy is only 15–30% (2).
Programmed death 1/anti-programmed death ligand 1 (PD-1/L1) as an inhibitory pathway detected in various malignant tumors that regulates the function of autoimmunity to against tumors, therefore the inhibitors of PD-1/L1 pathway started a new era of cancer treatment (3,4). Except for FDA approved PD-1/L1 inhibitors in NSCLC (nivolumab, pembrolizumab, atezolizumab and durvalumab), many other agents with ongoing clinical trials also demonstrated satisfactory efficacy and safety for advanced NSCLC.
Recently, a series of randomized controlled trials (RCTs) demonstrated significant clinical benefits in front line treatment for NSCLC using PD-1/L1 inhibitors, including longer time-to-event outcomes and less side effects (5-7). However, no head-to-head clinical trial has ever compared which PD-1/L1 agent or strategy is the optimal choice. In addition, there is no study comparing the efficacy of PD-1/L1 inhibitors according to PD-L1 expression.
To address this gap, we performed a Bayesian network meta-analysis (NMA) of RCTs to compare these strategies for untreated advanced NSCLC, attempting to identify the optimal treatment.
Methods
Search strategy
Systematic search was conducted through PubMed and Web of Science to select RCTs before 1 January 2020. Keywords: NSCLC, first-line, front-line, PD-1, PD-L1, atezolizumab, caremlizumab, nivolumab, pembrolizumab and the name of other PD-1/L1 inhibitors. We also screened EMSO, ASCO, and WCLC of recent years to avoid missing updated data. This NMA was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the PRISMA extension statement for NMAs.
Selection criteria
The inclusion criteria of this NMA were as follow: (I) RCTs; (II) PD-1/L1 inhibitors as first-line therapy; (III) comparison between chemotherapy and immunotherapy or their combinations; (IV) completed outcomes. Exclusion criteria: (I) studies included patients that received other therapies in front-line other than chemotherapy or immunotherapy; (II) studies included patients with EGFR, ALK or other sensitive mutations; (III) systematic reviews, meta-analyses, case reports, letters or non-English documents.
Data extraction and quality assessment
Two researchers (Guo Lin and Hengrui Liang) independently conducted the data extraction and the following data were summarized: author, publication year, phase of trials, treatments, number of patients, histology type, gender, age, smoke status, Eastern Cooperative Oncology Group (ECOG) score, objective response rate (ORR), time-to-event outcomes included overall survival (OS) and progression-free survival (PFS), and side effects were more than grade 3 treatment-related severe adverse events (tr-SAE).
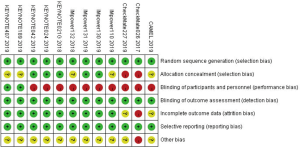
The quality assessment was performed using Cochrane risk of bias tools from 7 perspectives: (I) random sequence generation; (II) allocation concealment; (III) blinding of participants and personnel; (IV) blinding of outcome assessment; (V) incomplete outcome data; (VI) selective reporting; (VII) other bias. Disagreements were resolved via discussion among authors.
Statistical analysis
We included all direct and indirect data to compare the efficacities of different therapies. OS and PFS were primary outcomes. ORR and the incidence rate of tr-SAE were secondary outcomes. Hazard ratios (HR) for OS and PFS, odds ratios (OR) for ORR and the incidence rate of tr-SAE were calculated.
Open BUGS (version 3.2.3) software was applied to perform Bayesian network-meta analysis in random-effect model. Based on noninformative uniform and normal prior distributions, we generated 3 chains and used 50,000 iterations with 20,000 burn-ins for each chain (the thinning interval was 10). Moreover, this software can identify the probability of each regimen to be ranked the best, second best, third best, etc. Based on the surface under the cumulative ranking curves (SUCRA) for aforementioned endpoints, we showed them in ranking plots using Microsoft Excel.
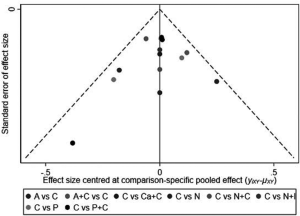
Subgroup analysis was performed according to PD-L1 expression. Network plots were completed based on the connection between eligible trials according to the number of trials and sample size. Traditional pairwise meta-analyses (PWMA) were applied to compare multiple trials and control treatment simultaneously. Heterogeneity was evaluated by chi-square test and I-square test, using random-effect model if P values <0.05 otherwise fixed-effect model was used. Funnel plots, Begg’s and Egger’s test were applied for testing publication bias. To ensure the reliability of NMA, sensitive analysis was performed by excluding phase II trials with a small sample size and subgroup analyses also performed in sensitivity analysis. All tests were two-sided, with an a-level of 0.05.
Results
Study selection and characteristics
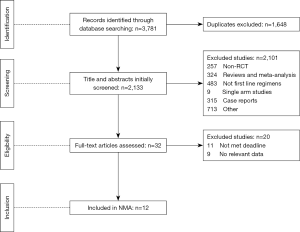
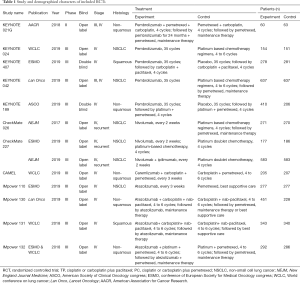
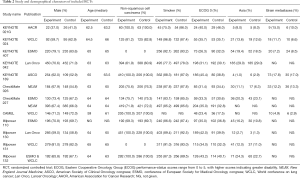
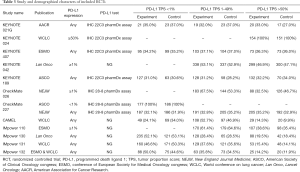
We identified 3,781 records from online databases and international conferences. After excluding duplicates and screening for titles/abstracts, 32 studies were reviewed for full-text assessment. Eventually, 12 studies met the selection criteria (Figure 1). All updated data were used in our pooled analysis. The detailed information of study characteristics was summarized in Tables 1-3. Overall, 7,490 patients were enrolled in 9 different treatment strategies (8-19): chemotherapy, pembrolizumab, nivolumab, atezolizumab, pembrolizumab plus chemotherapy, nivolumab plus chemotherapy, atezolizumab plus chemotherapy, caremlizumab plus chemotherapy and nivolumab plus ipilimumab.
Full table
Full table
Full table
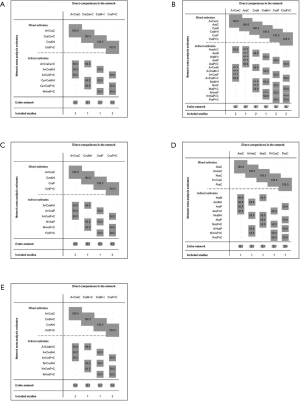
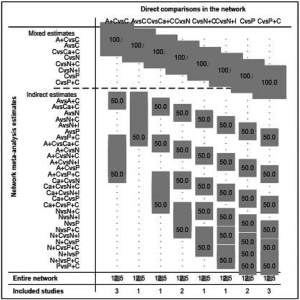
In total, the networks included 9 treatment strategies for PFS, OS, ORR and tr-SAE. All studies but KEYNOTE-021G (phase II clinical trials) were multi-center phase III clinical trials. All studies were double-arm trials, except for CHECKMATE-227 (four arms): nivolumab, nivolumab plus chemotherapy, nivolumab plus ipilimumab and chemotherapy. See Figure S1 for detailed results of the bias assessment.
NMA in PD-L1 non-selective NSCLC patients
For the PD-L1 expression non-selective population, 5 treatments reported OS (Figure 2A). All combination treatments were significantly better than chemotherapy for prolonging OS, except for caremlizumab plus chemotherapy (HR =0.72, 95% CI: 0.49 to 1.04). Among these combination strategies, pembrolizumab plus chemotherapy performed significant better OS than atezolizumab plus chemotherapy (HR =0.75, 95% CI: 0.58 to 0.95) (Figure 2B). Forest plots and contribution plots were presented in Figures S2-S6.
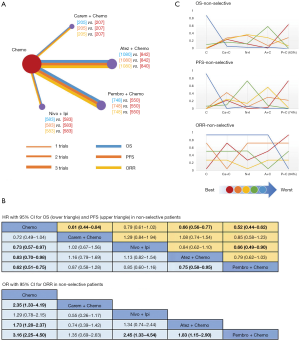
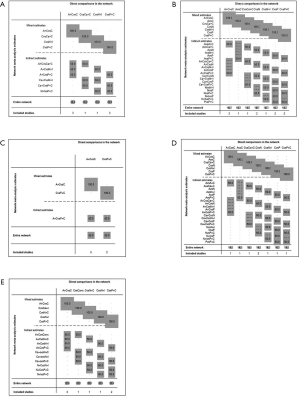
In this subgroup PFS was assessed for 5 treatments (Figure 2A). All combination therapies were significantly better than chemotherapy on PFS, except for nivolumab plus ipilimumab (HR =0.79, 95% CI: 0.61 to 1.02). Additionally, pembrolizumab plus chemotherapy performed notably longer PFS than nivolumab plus ipilimumab (HR =0.66, 95% CI: 0.49 to 0.90) and was equal to atezolizumab plus chemotherapy (HR =0.79, 95% CI: 0.62 to 1.03) and caremlizumab plus chemotherapy (HR =0.85, 95% CI: 0.58 to 1.23) (Figure 2B).
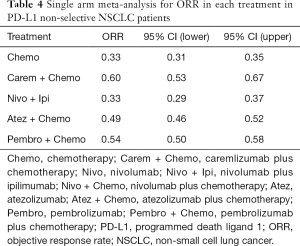
Table 4 showed absolute value of pooled ORR of each treatment via single arm meta-analysis. All combination therapy significantly increased the ORR compared with chemotherapy alone expect for nivolumab plus ipilimumab (OR =1.29, 95% CI: 0.78 to 2.15). Specially, ORR in pembrolizumab plus chemotherapy was significantly higher than that in atezolizumab plus chemotherapy (OR =1.83, 95% CI: 1.15 to 2.90), nivolumab plus ipililumab (OR =2.45, 95% CI: 1.33 to 4.54) but similar with caremlizumab plus chemotherapy (OR =1.35, 95% CI: 0.69 to 2.63) group (Figure 2B).
Full table
Bayesian ranking profiles (Figure 2C) suggested that pembrolizumab plus chemotherapy was most likely to be ranked as first for OS (probability =63%), PFS (probability =74%) and ORR (probability =94%) in PD-L1 expression non-selective NSCLC patients.
Subgroup analysis according to PD-L1 expression
PD-L1 TPS ≥50%
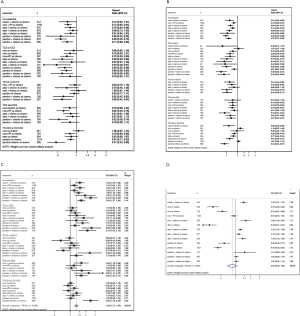
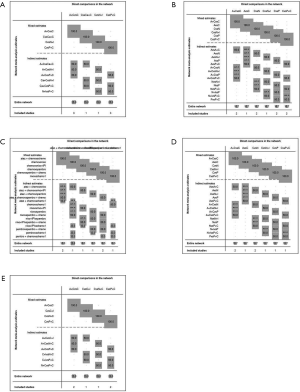
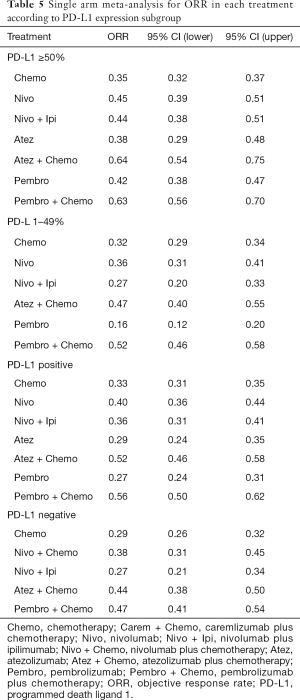
8 treatments were included in the PD-L1 ≥50% population (Figure 3). When using mono-immunotherapy, pembrolizumab (HR =0.67, 95% CI: 0.51 to 0.88) and atezolizumab (HR =0.59, 95% CI: 0.36 to 0.95) would significantly prolong OS compared with chemotherapy. Only pembrolizumab had significant benefit compared with chemotherapy according to PFS (HR =0.67, 95% CI: 0.45 to 0.94). No significant survival or response difference was found among monotherapy of all PD-1/L1 inhibitors (pembrolizumab vs. atezolizumab vs. nivolumab). As for chemoimmunotherapy, pembrolizumab plus chemotherapy and atezolizumab plus chemotherapy were better than chemotherapy both in OS and PFS. All chemotherapy-based combination strategies performed similar in survival comparison (Figure 4). Table 5 showed absolute value of pooled ORR of each treatment via single arm meta-analysis. As for ORR comparison, Pembrolizumab plus chemotherapy was equal to atezolizumab plus chemotherapy (OR =1.79, 95% CI: 0.68 to 4.74) and superior to any other treatments.
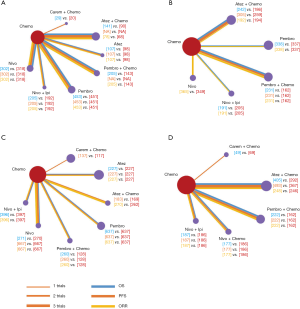
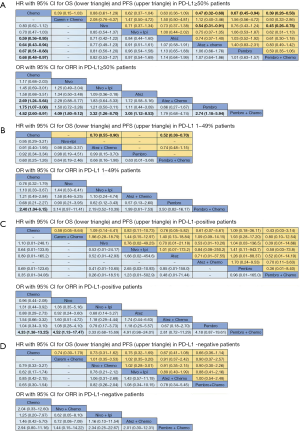
Full table
Bayesian ranking profiles (Figure 5) suggested atezolizumab alone was most likely to be ranked as first to offer best OS (probability =41%), caremlizumab plus chemotherapy had the highest possibility to offer best PFS (probability =45%) and pembrolizumab plus chemotherapy was the best possible treatment for ORR (probability =95%).

PD-L1 TPS 1–49%
6 treatments were included for accessing the best strategy in PD-L1 1–49% NSCLC patients (Figure 3B). All included treatments showed similar OS. For PFS, pembrolizumab plus chemotherapy was similar to atezolizumab plus chemotherapy (HR =0.74, 95% CI: 0.48 to 1.15) and were both better than chemotherapy. Only pembrolizumab plus chemotherapy showed higher ORR than chemotherapy (HR =2.40, 95% CI: 1.04 to 6.15) (Figure 4B).
According to Bayesian ranking profiles (Figure 5), the combination of pembrolizumab and chemotherapy was most likely to be ranked as first to offer best OS (probability =65%), PFS (probability =91%) and ORR (probability =92%).
PD-L1 TPS >1%
For PD-L1 positive expressed advanced NSCLC patients, 8 treatments were included in analysis (Figure 3C). All included regimens showed similar efficacy in this sub-population according to OS and PFS. Pembrolizumab plus chemotherapy significantly increase ORR than nivolumab (HR =4.52, 95% CI: 1.13 to 17.47) and chemotherapy (HR =4.33, 95% CI: 1.38 to 13.25) (Figure 4C).
Bayesian ranking profiles (Figure 5) indicated that the pembrolizumab plus chemotherapy was most likely to be the best regimen for increasing OS (probability =34%), PFS (probability =46%) and ORR (probability =94%).
PD-L1 TPS <1%
6 treatments were included in PD-L1 non-expressed population (Figure 3D). All treatments were equivalence according to OS, PFS and ORR (Figure 4D).
Bayesian ranking profiles (Figure 5) suggested the combination of nivolumab and ipilimumab was the most possible therapy to be ranked as first for OS (probability =45%); pembrolizumab plus chemotherapy had the greatest possibility to favor PFS (probability =25%) and ORR (probability =82%).
Safety analysis
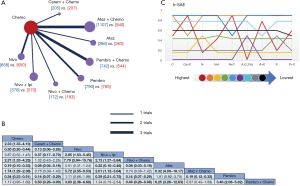
All 12 studies including 9 treatments were involved in tr-SAE NMA (Figure 6A). Table 6 showed the pooled ORR of each treatment via single arm meta-analysis.
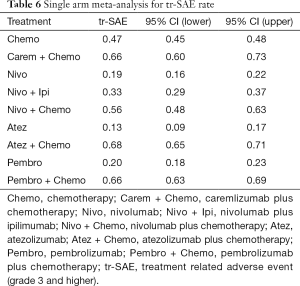
Full table
All mono-immunotherapy had significant lower tr-SAE than chemotherapy. No significant difference was found among mono-immunotherapies (pembrolizumab vs. atezolizumab vs. nivolumab). All chemotherapy-based regimens had higher tr-SAE than chemotherapy except for pembrolizumab plus chemotherapy (OR =1.17, 95% CI: 0.85 to 1.69). In addition, the tr-SAE of pembrolizumab plus chemotherapy lower than other chemotherapy-based regimens expect for atezolizumab plus chemotherapy (OR =0.67, 95% CI: 0.43 to 1.09) (Figure 6B).
The ranking outcomes (Figure 6C) suggested that atezolizumab monotherapy had the lowest opportunity (probability =2.2%) to confront tr-SAE among all mono-immunotherapies. Pembrolizumab plus chemotherapy had the lowest possibility (probability =59%) among chemotherapy-based combination therapies.
Sensitivity analysis and publication bias
We performed sensitivity analysis after excluding KEYNOTE-021G trials, which is a small sample size phase II clinical trial. Nine treatments for 7,367 untreated NSCLC patients were included for analysis. The results were stable and were similar to main analysis after excluding KEYNOTE-021G. Outcomes of node-splitting analysis indicated that there is no inconsistency exist then we did not conduct NMA in consistency model. Begg’s and Egger’s test demonstrated no obvious publication bias existed (Figures S7-S18).

Discussion
PD-1/L1 inhibitors are now widely used in solid tumors, including NSCLC. Meanwhile, some agents are rapidly promoted to first-line treatment. However, systematic comparisons among treatment strategies are lacking. Our research provided evidence to fill this gap and accurate clinical application of PD-1/L1 inhibitors in first line treatment of NSCLC.
Basing on 12 RCTs, the results of this NMA suggested that the combination of pembrolizumab and chemotherapy had potential advantage in terms of OS, PFS, ORR in most sub-populations. Moreover, patients with high PD-L1 expression obtain more advantages, this conclusion was also in accordance with previous analyses (20,21). Besides the combination of pembrolizumab and chemotherapy, our research provided valuable evidence for the effectiveness of other treatment regimens. For instance, according to the OS of PD-L1 negative patients, Bayesian ranking profiles suggested that the combination of nivolumab and ipilimumab was the most possible advantageous therapy to be ranked at first (probability =45%). More studies should be performed to validate and explore the optimal situation to use the doublet immunotherapy agents.
In this research, we found different PD-1/L1 agents had different efficacy in monotherapy and combination therapy. Several possible reasons might attribute to this phenomenon. There are two considerations from the perspective of drugs’ mechanism. (I) The different mechanisms between PD-1 and PD-L1 inhibitors. Although both inhibitors have therapeutic effect by blocking the binding of PD-1 to PD-L1, PD-1 inhibitors and PD-L1 inhibitors target different binding sites. Some researches indicated that PD-L1 inhibitors could produce a stronger immune response than PD-1 inhibitors due to that they could block both PD-L1/PD-1 and PD-L1/B7-1 pathway (22-25). Although PD-1 inhibitors can also bind to PD-L2, the function of PD-L2 in cancer immunosuppression does not seem to be important. Otherwise, PD-1 is expressed on a variety of immune cells, such as monocytes, T cells, B cells, dendritic cells, and tumor-infiltrating lymphocytes. However, PD-L1 is expressed in tumor cells and antigen presenting cells (APCs) (26). Therefore, the number of different cells and the expression of PD-1/PD-L1 may affect the efficacy. (II) The different bio-structure and binding sites among different PD-1/PD-L1 inhibitors. Although PD-1/L1 inhibitors work by binding to PD-1/L1 on tumors or somatic cells, their binding sites and mechanisms are different. For PD-1 inhibitors, nivolumab bound to a completely different area compared with pembrolizumab. The two antibodies bind PD-1 in two different orientations with steric clash. The binding surface of nivolumab on PD-1 is close to that of pembrolizumab, but they do not overlap (24). For PD-L1 inhibitors, atezolizumab and BMS-963559 bind to the upper side close to the N-terminus of PD-L1. In contrast, durvalumab and avelumab bind rather perpendicularly to PD-L1, which means that different drugs will take different forms when they combine to PD-L1 (27). However, some studies revealed that the efficacy of PD-1/L1 inhibitors was drug-independent (28). So, whether the difference of bio-structure and binding sites will play a significant role in the different efficacy of various PD-1/L1 inhibitors is unclear.
There are also another two considerations on design of clinical trials to explain the clinical difference of these immunological agents. (I) Heterogeneity of combination regimens. The included trials were heterogeneous not only in terms of type of checkpoint inhibitors, but also for the combined chemotherapy. For different chemotherapy, we should also consider the differences in synergy with immunotherapy. The binding kinetics of therapeutic antibodies is one of the most important determinants for the ultimate therapeutic function. Different structures in drugs can aid in controlling the surface complementarity of the interface between antibodies and immune checkpoints (28). For chemotherapy, it can stimulate the antigenicity and immunogenicity of malignant cells or increase their susceptibility to immune attacks and may be advantageously combined with immunotherapeutic regimens designed to activate immune effectors or to inhibit immunosuppressive mechanisms. However, not all chemotherapy regimens have the same effect which will cause different efficacy among various combination therapy regimens (29). (II) The difference of characteristics of eligible patients in each RCT. The clinical characteristics of eligible patients were different, in terms of smoking history, region, the proportion of different tumor histology, differences in PD-L1 assays, scoring and cutoff points employed in trials conducted by different study sponsors, and the impact of these distinctions cannot be ignored.
We acknowledged several limitations in our research. First, subgroup population only based on the PD-L1 expression may still lack the accuracy. Some current studies pointed out that the prediction of PD-L1 expression on the efficacy of PD-1/L1 inhibitors is not applicable to all types of patients in NSCLC (30,31). Second, we used high-graded adverse events to assess toxicity generally instead of overall adverse events such as low-grade neutrophil count, febrile neutropenia and so on. Therefore, although the incidence of high-level side effects is reduced, detailed slide side effects may not be reduced but increased for some therapy regimens. Third, all comparisons between therapies in our research are indirect, so more direct comparison data may be needed to support our conclusions. Lastly, some trials are still in progress and complete data cannot be included to participate in the full analysis. Therefore, more clinical trial results are needed to support our continued research.
Conclusions
These results indicated that pembrolizumab plus chemotherapy might be associated with the best therapeutic efficacy in first-line treatment for major population of NSCLC patients.
Acknowledgments
Funding: This study is supported by the following funding: National Key R&D Program of China (No. 2017YFC0907900/2017YFC0907903); China National Science Foundation (Grant No. 81871893); Key Project of Guangzhou Scientific Research Project (Grant No. 201804020030); High-level university construction project of Guangzhou medical university (Grant No. 20182737, 201721007, 201715907, 2017160107); National key R & D Program (Grant No. 2017YFC0907903 & 2017YFC0112704) and the Guangdong high level hospital construction “reaching peak” plan.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.02.14). WL serves as an unpaid Associate Editor-in-Chief of Translational Lung Cancer Research. HL serves as an unpaid Section Editor of Translational Lung Cancer Research from Jan 2020 to Dec 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Arulananda S, Mitchell P. Elderly patients with stage III NSCLC survive longer when chemotherapy is added to radiotherapy—fortune favours the bold. Transl Lung Cancer Res 2018;7:S388-92. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Veluswamy P, Bruder D. PD-1/PD-L1 pathway inhibition to restore effector functions in exhausted CD8+ T cells: chances, limitations and potential risks. Transl Cancer Res 2018;7:S530-7. [Crossref]
- Afzal MZ, Dragnev KH, Shirai K. An extended overall survival analysis of pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced non-squamous non-small cell lung cancer. Ann Transl Med 2019;7:S53. [Crossref] [PubMed]
- Theelen WS, Baas P. Pembrolizumab monotherapy for PD-L1 ≥50% non-small cell lung cancer, undisputed first choice? Ann Transl Med 2019;7:S140. [Crossref] [PubMed]
- Ackermann CJ, Reck M, Paz-Ares L, et al. First-line immune checkpoint blockade for advanced non-small-cell lung cancer: Travelling at the speed of light. Lung Cancer 2019;134:245-53. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Barlesi F, Nishio M, Cobo M, et al. IMpower132: Efficacy of atezolizumab (atezo) plus carboplatin (carbo)/cisplatin (cis) plus pemetrexed (pem) as 1L treatment in key subgroups with stage IV non-squamous non-small cell lung cancer (NSCLC). Ann Oncol 2018;29:743. [Crossref]
- Borghaei H, Langer CJ, Gadgeel S, et al. 24-Month Overall Survival from KEYNOTE-021 Cohort G: Pemetrexed and Carboplatin with or without Pembrolizumab as First-Line Therapy for Advanced Nonsquamous Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:124-9. [Crossref] [PubMed]
- Zhou C, Huang Y, Zhou J, et al. OA04.03 A Randomized Phase 3 Study of caremlizumab plus chemotherapy as 1st Line Therapy for Advanced/Metastatic Non-Squamous Non-Small Cell Lung Cancer. World Conference on Lung Cancer 2019;14:S215-6.
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Spigel D, Giaccone G, Reinmuth N, et al. LBA78-IMpower110: Interim OS Analysis of a Phase III Study of Atezolizumab vs Platinum-Based Chemotherapy as 1L Treatment in PD-L1-selected NSCLC. World Conference on Lung Cancer 2019;30:v915.
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab plus carboplatin plus paclitaxel or nab-paclitaxel vs carboplatin plus nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36:2. [Crossref]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Reck M, Schenker M, Lee KH, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer 2019;116:137-47. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Dafni U, Tsourti Z, Vervita K, et al. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 2019;134:127-40. [Crossref] [PubMed]
- Rossi A, Noia VD, Gkountakos A, et al. PD-L1 for selecting non-small-cell lung cancer patients for first-line immuno-chemotherapy combination: a systematic review and meta-analysis. Immunotherapy 2019;11:921-30. [Crossref] [PubMed]
- Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27:111-22. [Crossref] [PubMed]
- Butte MJ, Pena-Cruz V, Kim MJ, et al. Interaction of human PD-L1 and B7-1. Mol Immunol 2008;45:3567-72. [Crossref] [PubMed]
- Yang J, Riella LV, Chock S, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol 2011;187:1113-9. [Crossref] [PubMed]
- You W, Liu M, Miao JD, et al. A Network Meta-analysis Comparing the Efficacy and Safety of Anti-PD-1 with Anti-PD-L1 in Non-small Cell Lung Cancer. J Cancer 2018;9:1200-6. [Crossref] [PubMed]
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol 2017;8:561. [Crossref] [PubMed]
- Lee HT, Lee JY, Lim H, et al. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci Rep 2017;7:5532. [Crossref] [PubMed]
- Fessas P, Lee H, Ikemizu S, et al. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol 2017;44:136-40. [Crossref] [PubMed]
- Zitvogel L, Galluzzi L, Smyth MJ, et al. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013;39:74-88. [Crossref] [PubMed]
- Zhang B, Liu Y, Zhou S, et al. Predictive effect of PD-L1 expression for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatment for non-small cell lung cancer: A meta-analysis. Int Immunopharmacol 2020;80:106214. [Crossref] [PubMed]
- Raphael J, Batra A, Boldt G, et al. Predictors of Survival Benefit From Immune Checkpoint Inhibitors in Patients With Advanced Non-small-cell Lung Cancer: A Systematic Review and Meta-analysis. Clin Lung Cancer 2020;21:106-113.e5. [Crossref] [PubMed]