Role of chest radiographs in early lung cancer detection
Introduction
In 2018, Siegel et al. predicted that the number of newly diagnosed lung cancers that year would be approximately 234,030, making it the second most common cancer. Moreover, they estimated the number of deaths from this condition in 2018 would be approximately 154,050 (25.3% of total cancer deaths), making it the most fatal form of cancer (1).
Despite the risk of overdiagnosis, false positives, radiation exposure, and unnecessary studies, low-dose computer tomography (LDCT) screening of at-risk patients has offered positive outcomes for lung cancer mortality. The United States preventive services task force recommends annual LDCT screening for people aged 55 to 80 years with a 30 pack-year smoking history and a history of smoking within the last 15 years (2,3).
However, this does not mean all lung cancers can be detected with LDCT (4). Additionally, although the amount of radiation exposure in LDCT is 1/6 of that in regular CT (5), the potential health risk from this exposure is still significant (6). Furthermore, abundant resources and manpower are also needed to operate this screening system.
Conversely, chest radiographs (CXRs) are one of the most commonly utilized diagnostic tools for chest diseases in clinical practice. The technology is easy to operate and the procedure has a relatively low level of radiation exposure; thus, it is the standard diagnostic tool for respiratory illnesses. Also, it imposes less of a burden on radiologists than other, more sophisticated diagnostic imaging tools.
However, CXRs are not without their shortcomings. CXRs are often performed by personnel that do not specialize in radiology. Therefore, they are more prone to misinterpretation. Of all misdiagnosed lung cancer cases, 90% utilized CXRs and 10% used CTs and other diagnostic tools (7). Misinterpreting images can result in delayed diagnosis and more negative clinical outcomes. Identifying lesions early while maintaining accuracy is the key to improving lung cancer survival (8,9).
Multiple studies have suggested that using CXRs in lung cancer screening may improve survival. For instance, Strauss et al. reported findings from randomized controlled trials that show CXR screening can improve lung cancer survival as cancers are diagnosed at earlier stages (8,9). Another large population-based cohort study showed an 18% reduction in lung cancer mortality with CXR screening in at-risk populations (10). Furthermore, a case-control study found that lung cancer mortality was reduced by more than 20% with CXR screening (11). These studies suggest that CXRs can be significantly beneficial for the screening of lung cancer.
In this study, we examine whether retrospective observation of lung cancer patients confirmed by pathology can elucidate significant radiological abnormalities earlier than when diagnoses were made. We also introduce a new comparison method for CXR interpretation in lung cancer patients. We hypothesize that side-by-side comparisons of cropped CXRs will improve abnormal lesion detection.Methods
CXRs were collected from 1,500 lung cancer patients who presented to Uijeongbu St. Mary’s Hospital from 2006 to 2016 and whose diagnoses were confirmed by pathology. Excluding patients who were accurately diagnosed upon their first visit and selecting for a wide variety of lesion locations, we compiled radiographs from 50 cases. Cases were selected only from those that presented first to Uijeongbu St. Mary’s Hospital without a diagnosis or suspicion of lung cancer. Cases that were transferred from other hospitals were only considered if the lung cancer was an incidental finding; overall, only patients who had initial CXRs not showing lung cancer or those for which cancer was an incidental finding were included. Patients who were diagnosed retrospectively were selected by 2 radiologists, each with 33 years of clinical experience. We also added 5 normal sets of CXRs as controls. These patients were cancer-free for at least 3 years; two were completely normal and 3 developed chronic obstructive pulmonary disease. For research participants, attending physicians who were board certified in pulmonology were recruited from 9 university hospitals in the Republic of Korea. To eliminate bias, no physicians were recruited from Uijeongbu St. Mary’s Hospital. The study was approved by the Uijeongbu St. Mary’s Hospital Institutional Review Board (#UC17EESI0128). Informed, written consent was obtained from each participant.
For each case, we collected the CXRs obtained until the chest CT that was used to diagnose lung cancer. We selected 6 CXRs with the earliest being a “normal” image, if possible. Subsequent radiographs were selected from those that (I) we suspected showed lesions and (II) were equally spaced chronologically. In cases that had fewer than 5 CXRs that showed lesions, additional earlier CXRs were used as supplements. In cases with more than 6 CXRs, 6 radiographs were selected between when the patient was diagnosed and the last normal CXR, with chronological spacing between the radiographs as equally as possible. Radiographs that were obtained more than 3 years before the next CXR were excluded to minimize potentially severe contrasts. Radiograph selection and confirmation of lesion location with CT were performed by 2 radiologists each with 33 years of clinical experience.
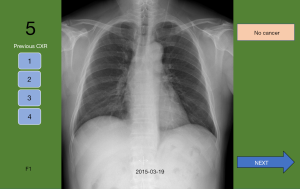
All images, after removal of patient identifiers, were converted to digital files in the jpg format at maximum quality and scaled to 100%. A computer programmer designed the examination program used to evaluate the participants. Images for each case were arranged in chronological order. As in a previously published study, the lesion areas on each image were outlined by freeform outlining using the Adobe Captivate 9 ‘hot spot’ technique (Adobe systems Inc., San Jose, CA, USA) (12). Outlines were made 100% transparent to prevent participants from noticing them. The last image in each case was designated as ‘0’ while other images were designated with numbers that represented the number of days between when those images were taken and when image 0 was taken. If the participant placed the mouse pointer correctly within the outline and clicked, the image’s designated number was noted. If the participant clicked in an incorrect area, an ‘X’ was noted but was not visible to the participant. If the participant thought there were no noticeable lesions on the image, he or she could click a button labeled ‘N’. Participants were allowed to click on each image only once.
Participants were allowed to navigate between the slides using arrows on the screen but could not see chronologically later radiographs until they clicked to indicate their designation on the most recent image (Figure 1). This design emulated the conditions used in actual clinical settings, wherein physicians were able to compare recent CXRs with previous ones, but which eliminated the systemic limitations, such as time constraints and other distractors (A). After the reviewing the last image of a case, a slide showed a compilation of the cropped regions of the reviewed images with the lesion. Participants were then asked to identify the image which made them primarily diagnose cancer (DX) and first suspect a lesion (E) (Figure 2). The dates of all cases of early detection were measured as that furthest away from the day of actual diagnosis. After recording DX and E, all finalized answers were presented in a portable document format (PDF) file (Figure 3).
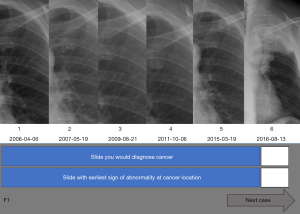
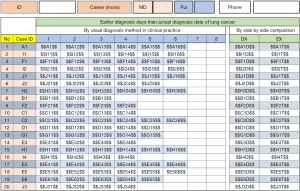
Lesion diameters, as shown on the CXRs, and the number of days preceding the date of actual diagnosis (F) were recorded. The variables (A, DX, E, F) were compared at each TNM classification of malignant tumors stage using the Wilcoxon rank sum test. Continuous data were reported as mean ± standard deviation, and categorical data as numbers and percentages. Missing data were treated using the Last Observation Carried Forward (LOCF) method.
Further performance comparisons between CXRs showing lesions in ‘hidden’ areas (paraaortic area, paratracheal region, retrocardiac region, subdiaphragmatic region, apices, and hilum area) and in ‘open’ areas (right upper lobe, right middle lobe, right lower lobe, left upper lobe, and left lower lobe) were performed. Also, we examined whether differences in the amount of clinical experience, measured by the number of months the physician was a medical doctor vs. a specialized pulmonologist, had an impact on performance. All statistical analyses were conducted by SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and a P value below 0.05 was considered statistically significant. Data for these comparisons were expressed as means ± standard deviations or as medians with interquartile ranges.
Results
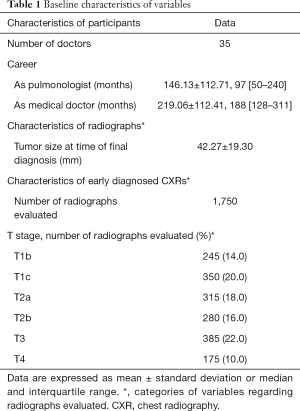
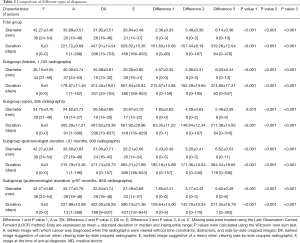
Thirty-five physicians participated in this study. Participants had 146.13±112.71 months of clinical experience as pulmonologists. CXRs compiled from 50 cases yielded 1,750 lesion evaluations at various stages of lung cancer (Table 1). Data analysis showed participants performed significantly better without systemic constraints, making diagnoses on average 221.72±9.69 days earlier. Furthermore, cropping of relevant areas on the CXRs had a significant, positive impact on cancer detection. Cropping of images to focus on the same area on multiple CXRs further expedited diagnosis by 161.83±10.66 days, with a reduction of the lesion size by 2.36±0.33 mm at the time of diagnosis (P<0.05). This benefit was evident regardless of the lesion location or the physician’s length of clinical experience (Table 2). Thus, cropping allowed participants to detect lesions at significantly smaller diameters and significantly earlier than when a cancer diagnosis would have been made (P<0.001). Comparisons between ‘hidden’ and ‘open’ areas showed significantly better performance with ‘open’ areas at all stages (P<0.001) (Tables 2,3). Comparisons between different lengths of clinical experience showed no significant difference between the two subgroups at all stages (Tables 2,4).
Full table
Full table

Full table

Full table
Discussion
This study demonstrated that CXRs can be used to diagnose lung cancer earlier and that a side-by-side comparison of cropped images can enhance lung cancer detection. On average, participants were able to identify lesions 221.72 days before actual diagnoses were made using this approach. All radiographs presented were subject to the same independent analysis as would be expected in real-life situations, with no second opinion from radiologists or patients present to provide additional information. As improved performance was observed with less systemic constraints, it is plausible that better CXR reading can be achieved with improvements to the health care system. Such improvements would allow physicians to evaluate these images with fewer distractions and less time pressure. Additionally, assessing cropped CXRs displayed side-by-side offered further significant improvements, with participants detecting lesions 629.32 days earlier on average. These benefits seemed to exist across different anatomical regions and different lengths of clinical experience. This suggests that the development of software that can display all relevant CXRs side-by-side while allowing physicians to crop them all simultaneously would have significant potential advantages. These benefits will likely improve lung cancer detection at all levels of clinical practice.
Role of CXRs
CXRs are used as screening tests for chest diseases as well as other disorders. They have a low cost, can be used conveniently at bedside, have low radiation exposure, and provide an abundance of information that is useful in follow-up studies. Although survival benefits are much higher with LDCT and the advantages of screening with CXRs are still being investigated (8,9,13-16), the possible merit of CXRs as a screening tool cannot be ignored.
In 2015, 24,267 patients in the Republic of Korea were diagnosed with lung cancer. Among them, 13,366 (55%: 9,868 males and 3,498 females) were aged between 55 and 75 years (17). A significant portion of the patient population does not meet the indication criteria for annual LDCT screening as it focuses on at-risk and symptomatic patients. Furthermore, financial difficulties, both in developed and underdeveloped countries, as well as patient aversion to radiation exposure can inhibit LDCT utilization. Considering these factors, there is a potential for increased CXR utilization as an alternative screening method.
Notably, with the commencement of the campaign for increased lung cancer awareness, there has been an increase in the utilization of CXR and in the proportion of lung cancer cases diagnosed in the earlier stages (proportion of patients diagnosed with stages I & II lung cancer: before campaign, 26.5% vs. during campaign, 35.3%), as well as a reduction in the number of lung cancer cases diagnosed in the later stages (absolute number of patient diagnosed with stage III & IV lung cancer: before campaign, 1,254 vs. during campaign, 1,137) (18). Although the correlation between these observations has not yet been proven, it is a plausible conjecture that the increase in CXR screening contributed to this phenomenon. Our study is notable in that it demonstrates that CXRs are a potentially effective tool in lung cancer screening and confirms previous, retrospective findings in a controlled setting. Furthermore, we show that side-by-side comparisons enhanced by purposeful cropping can amplify its benefits. Hence, the addition of a simple feature to existing image viewing software can greatly enhance the ability of physicians to diagnose lung cancer earlier.
It is important to note that, although LDCT screening remains the standard screening method, it has certain shortcomings. Despite adequate LDCT screening, some lung cancers may still be missed (19). Importantly, the total annual cost between LDCT screening and CXR screening is not significantly different (20). Thus, because CXR is performed in much larger proportion of cases than LDCT, it is important for physicians and radiologists to accurately read CXRs. Well-organized education in CXR interpretation may improve accuracy (21).
Misinterpretations in radiology
Approximately 1–4% of radiology reports are misinterpreted (22,23). This results in approximately 30% of abnormalities being missed in radiologic examinations (24). Diagnostic errors can be categorized into missed (no diagnosis made), false (incorrect diagnosis), or delayed (diagnosis delayed although sufficient information was available earlier) (24,25). Several factors are involved in diagnostic errors. ‘Hidden’ areas are locations on images where lesions are harder to see due to adjacent or overlapping structures; lesions can also be hard to find due to weak contrast or density. Furthermore, lesions can be missed due to observer fatigue, sleepiness, lack of adequate lighting, or time constraints. To help minimize errors, interpretation conditions or technical factors such as reading room light conditions, viewing distance, and monitor resolution must be improved (26).
According to Kundel et al., errors in reading radiographs are caused by scanning, recognition, and decision-making errors. Of the false negative errors they reported, 30% were by scanning, 25% were by recognition, and 45% were by decision-making (27). Kim et al. have categorized causes of errors as complacency, faulty reasoning, lack of knowledge, under-reading, miscommunication, technique, prior examination, history, lesion location, search satisfaction, complications, and report satisfaction (28). Improving CXR reading skills, either through education, removal of constraints, or through the development of better tools, could drastically reduce these errors. Our study was able to show that the removal of systematic constraints and utilization of better viewing tools results in significantly earlier lesion detection.
Moreover, the sensitivity and accuracy of CXR interpretation can be improved by increased knowledge and experience regarding how to read normal lines, spaces, stripes, and signs on relevant images in comparison to CTs. Errors can be minimized by improving search patterns or paying more attention to blind spot areas. New technologies, such as bone suppression software, dual-energy radiography, and computer-aided design systems are being developed to facilitate more accurate image assessments (29-32). It remains to be seen how these changes will help physicians interpret images.
Radiographs must be compared to previous images, consecutively from the least recent to the most recent. In this study, we compared the same regions in CXRs cropped and placed side-by-side, which increased participants’ sensitivity to lesion changes. However, such side-by-side, focused comparisons are difficult to achieve in real clinical situations. Further research will be needed to examine the utility of this method. As new technologies such as deep convolutional neural networks that allows automatic CXR comparisons to identify abnormalities have been recently developed, more research will be able to help determine its utility (33).
Relationship between missed diagnoses and prognoses
Although it is reasonable to assume delayed lung cancer detection will result in progression to higher stages, thereby having a negative impact on prognoses, it is difficult to say with certainty that delayed detection on CXRs has a direct negative impact on outcomes, as demonstrated by related previous studies that examined different patient populations with different methods (34-37). For instance, Quekel et al. examined cases of non-small cell lung cancer patients with nodular lesions and found that lesions were missed in 19% of the cases. They attributed this to a smaller median diameter of the lesions. The median delay in their study was 472 days, similar to that in our study. It is difficult to examine any changes in the N or M staging because CT was not performed in cases with missed cancer diagnosis (34). Because of the limitations in their study design, we cannot comment on its impact on cancer prognoses. However, the detection of cancers at smaller sizes with CXRs suggests that CXRs have the potential to play a significant role in the earlier detection of cancers.
Education regarding CXR interpretation is often limited to several hours during medical school. Even during residency and beyond, medical professionals often do not receive specialized training in reading CXRs. This may be attributable to the CXR marginalization with the increased use of CTs. Despite this deficiency, many clinicians make medical decisions based on CXR readings without assistance from radiologists. Therefore, routine education of medical professionals in reading CXRs would be beneficial.
Limitations
This study is limited by its relatively small sample size. Also, we were not able to compare the advantages of this study’s features between different medical specialties. Moreover, the potential benefits of cropped side-by-side comparisons of CXRs could have been further explored if the participant pool had been extended to include resident physicians. These variables should be further examined in subsequent studies.
Conclusions
All medical professionals must be able to interpret images accurately to maintain quality patient care. Education regarding CXR interpretation is conducted on a routine basis and can be achieved through various methods. However, education is often limited and capabilities between physicians vary, even within the field of pulmonology. All medical professionals, regardless of his or her specialty, should receive a more structured and specialized education on this subject. This study demonstrates that CXRs may have a significant role in earlier lung cancer detection. Also, comparing cropped radiographs side-by-side improves detection, highlighting a potential feature that could be implemented in image viewing programs. Future research is needed to examine whether the application of this method will help with earlier lung cancer detection and outcome.
Acknowledgments
We would like to thank Seunghyun Kim, M. Eng. (Cornell University Department of Computer Science) for developing the exam software used by participants and for assisting in the data analysis.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.04.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Uijeongbu Institutional Review Board (#UC17EESI0128). Written informed consent was obtained from each participant.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Hoffman RM, Sanchez R. Lung cancer screening. Med Clin North Am 2017;101:769-85. [Crossref] [PubMed]
- de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160:311-20. [Crossref] [PubMed]
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2018;68:297-316. [Crossref] [PubMed]
- Ruchalski K, Gutierrez A, Genshaft S, et al. The evidence for low-dose CT screening of lung cancer. Clin Imaging 2016;40:288-95. [Crossref] [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. [Crossref] [PubMed]
- White CS, Salis AI, Meyer CA. Missed lung cancer on chest radiography and computed tomography: imaging and medicolegal issues. J Thorac Imaging 1999;14:63-8. [Crossref] [PubMed]
- Strauss GM, Gleason RE, Sugarbaker DJ. Chest X-ray screening improves outcome in lung cancer. A reappraisal of randomized trials on lung cancer screening. Chest 1995;107:270S-279S. [Crossref] [PubMed]
- Strauss GM, Dominioni L. Chest X-ray screening for lung cancer; overdiagnosis, endpoints, and randomized population trials. J Surg Oncol 2013;108:294-300. [Crossref] [PubMed]
- Dominioni L, Poli A, Mantovani W, et al. Assessment of lung cancer mortality reduction after chest X-ray screening in smokers: a population-based cohort study in Varese, Italy. Lung Cancer 2013;80:50-4. [Crossref] [PubMed]
- Nakayama T, Baba T, Suzuki T, et al. An evaluation of chest X-ray screening for lung cancer in gunma prefecture, Japan: a population-based case-control study. Eur J Cancer 2002;38:1380-7. [Crossref] [PubMed]
- Kim J, Kim KH. Measuring the effects of education in detecting lung cancer on chest radiographs: utilization of a new assessment tool. J Cancer Educ 2019;34:1213-8. [Crossref] [PubMed]
- Wu GX, Raz DJ. Lung cancer screening. Cancer Treat Res 2016;170:1-23. [Crossref] [PubMed]
- Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the prostate, lung, colorectal, and ovarian (PLCO) randomized trial. JAMA 2011;306:1865-73. [Crossref] [PubMed]
- Manser RL, Irving LB, Byrnes G, et al. Screening for lung cancer: a systematic review and meta-analysis of controlled trials. Thorax 2003;58:784-9. [Crossref] [PubMed]
- Gossner J. Lung cancer screening-don't forget the chest radiograph. World J Radiol 2014;6:116-8. [Crossref] [PubMed]
- Korea Central Cancer Registry, National Cancer Center, Annual report of cancer statistics in Korea in 2015, Ministry of Health and Welfare, 2017. Available online: http://ncc.re.kr/cancerStatsList.ncc?searchKey=total&searchValue=&pageNum=1
- Kennedy MPT, Cheyne L, Darby M, et al. Lung cancer stage-shift following a symptom awareness campaign. Thorax 2018;73:1128-36. [Crossref] [PubMed]
- Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342-50. [Crossref] [PubMed]
- Gareen IF, Black WC, Tosteson TD, et al. Medical care costs were similar across the low-dose computed tomography and chest X-ray arms of the national lung screening trial despite different rates of significant incidental findings. Med Care 2018;56:403-9. [PubMed]
- Ogura A, Hayashi N, Negishi T, et al. Effectiveness of an e-learning platform for image interpretation education of medical staff and students. J Digit Imaging 2018;31:622-7. [Crossref] [PubMed]
- Siegle RL, Baram EM, Reuter SR, et al. Rates of disagreement in imaging interpretation in a group of community hospitals. Acad Radiol 1998;5:148-54. [Crossref] [PubMed]
- Borgstede JP, Lewis RS, Bhargavan M, et al. RADPEER quality assurance program: a multifacility study of interpretive disagreement rates. J Am Coll Radiol 2004;1:59-65. [Crossref] [PubMed]
- Lee CS, Nagy PG, Weaver SJ, et al. Cognitive and system factors contributing to diagnostic errors in radiology. AJR Am J Roentgenol 2013;201:611-7. [Crossref] [PubMed]
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf 2013;22:672-80. [Crossref] [PubMed]
- Shea FJ, Ziskin MC. Visual system transfer function and optimal viewing distance for radiologists. Invest Radiol 1972;7:147-51. [Crossref] [PubMed]
- Kundel HL, Nodine CF, Carmody D. Visual scanning, pattern recognition and decision-making in pulmonary nodule detection. Invest Radiol 1978;13:175-81. [Crossref] [PubMed]
- Kim YW, Mansfield LT. Fool me twice: delayed diagnoses in radiology with emphasis on perpetuated errors. AJR Am J Roentgenol 2014;202:465-70. [Crossref] [PubMed]
- Freedman M. State-of-the-art screening for lung cancer (part 1): the chest radiograph. Thorac Surg Clin 2004;14:43-52. [Crossref] [PubMed]
- Mazzone PJ, Obuchowski N, Phillips M, et al. Lung cancer screening with computer aided detection chest radiography: design and results of a randomized controlled trial. PloS One 2013;8:e59650. [Crossref] [PubMed]
- Schalekamp S, van Ginneken B, Heggelman B, et al. New methods for using computer-aided detection information for the detection of lung nodules on chest radiographs. Br J Radiol 2014;87:20140015. [Crossref] [PubMed]
- Al Mohammad B, Brennan PC, Mello-Thoms C. A review of lung cancer screening and the role of computer-aided detection. Clin Radiol 2017;72:433-42. [Crossref] [PubMed]
- Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology 2017;284:574-82. [Crossref] [PubMed]
- Quekel LG, Kessels AG, Goei R, et al. Miss rate of lung cancer on the chest radiograph in clinical practice. Chest 1999;115:720-4. [Crossref] [PubMed]
- Turkington PM, Kennan N, Greenstone MA. Misinterpretation of the chest x ray as a factor in the delayed diagnosis of lung cancer. Postgrad Med J 2002;78:158-60. [Crossref] [PubMed]
- Kashiwabara K, Koshi S, Ota K, et al. Outcome in patients with lung cancer found retrospectively to have had evidence of disease on past lung cancer mass screening roentgenograms. Lung Cancer (Amsterdam, Netherlands) 2002;35:237-41. [Crossref] [PubMed]
- Forrest JV, Friedman PJ. Radiologic errors in patients with lung cancer. West J Med 1981;134:485-90. [PubMed]