
Emergence of a HER2-amplified clone during disease progression in an ALK-rearranged NSCLC patient treated with ALK-inhibitors: a case report
Introduction
Anaplastic lymphoma kinase (ALK) rearranged disease account for 2–5% of non-small cell lung cancers (NSCLC) and it is an indication to administer ALK tyrosine kinase inhibitors (TKIs) in first and following lines of treatment of advanced stage patients (1). The therapeutic landscape of ALK-positive NSCLC is rapidly evolving thanks to the discovery of different molecules. Crizotinib (first-generation), ceritinib, alectinib, brigatinib (second-generation) and lorlatinib (third-generation) are currently available in clinical practice, while additional inhibitors are in development (e.g., ensartinib, entrectinib, repotrectinib) (2). The use of ALK-TKIs allows long survivals up to 5–7 years (3).
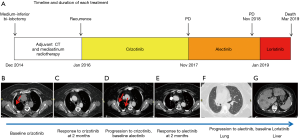
Whether ALK-inhibitors should be used in sequence and which is the best first are open issues in the scientific community (4). The main clinical issue, however, is the expected development of resistance mainly caused by the acquisition of ALK secondary mutations (5,6). ALK gene amplification can also cause resistance (7) as well as different by-pass ALK-independent mechanisms, such as epidermal growth factor receptor (EGFR), KIT, and insulin-like growth factor 1 receptor (IGF-1R) activation (5-8). Here, we present a case of an ALK-positive NSCLC patient treated with ALK-TKIs and progressed to alectinib treatment (Figure 1) via the emergence of a HER2-amplified clone (Figure 2).
Case presentation
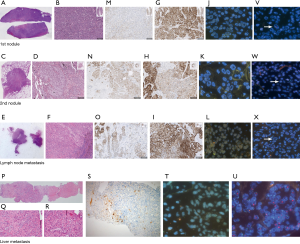
A 52-year-old never smoker woman, without a significant medical history, underwent in 2014 to medium-inferior bi-lobectomy for lung adenocarcinoma (Figure 1A). The pathological staging was T3N2, considering two neoplastic nodules in right inferior lobe (Figure 2A,B,C,D) and multiple hilo-subcarinal lymph nodal involvement (Figure 2E,F). In both nodules and in the metastasis, two distinct cell components were observed characterized by a solid and signet ring pattern. Molecular study of the nodules and the metastasis revealed KRAS and EGFR wild-type status. ALK (D5F3, Ventana BenchMark XT, OptiView AMP Kit) immunostaining was positive (Figure 2G,H,I) but fluorescent in situ hybridization (FISH, ALK Break Apart FISH, ABBOTT VYSIS) was negative (Figure 2J,K,L). Spatial variation in staining intensity (Figure 2G,H,I) was observed in all samples that was not caused by a fixation defect as it did not show zonal differences between peripheral (Figure 2M,N,O) and central areas of the tumor (Figure 2G,H,I). Conversely, the ALK staining pattern tended to segregate with the different cytotypes, the signet ring cells being mostly ALK-negative (Figure 2M,N,O).
Despite adjuvant chemotherapy and mediastinum radiotherapy, in January 2016, right pleura, mediastinal lymph node and bone recurrences occurred (Figure 1A,B). Based on the immunohistochemistry (IHC) ALK positivity, the patient received crizotinib treatment and radiotherapy on a L4 bone metastasis (Figure 1A). After 2 months of therapy, an improvement of the pleural involvement with stability of other lesions was observed (Figure 1C). Crizotinib was continued without relevant toxicity until November 2017 when progression in right pleura was observed (Figure 1D). A second-line treatment with alectinib was started with improvement of the pleura and stability at other sites (Figure 1E). In November 2018, after 1-year of alectinib therapy without adverse effects, lung and liver progression occurred (Figure 1F,G). A liver biopsy was obtained to study alectinib resistance. Written informed consent for molecular analysis was collected. The biopsy showed an adenocarcinoma (Figure 2P,Q,R) with homogeneous solid pattern, similar to that observed in the lung primary, but ALK-negative both by IHC and FISH (Figure 2S,T).
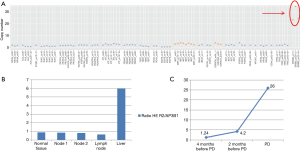
DNA was extracted from the liver biopsy and studied by next generation sequencing (NGS) (Solid Tumor Solution, Sophia Genetics, on MiSeq Platform, Illumina, USA). A HER2 gene amplification was observed (Figure 3A), confirmed with FISH analysis (Figure 2U) and digital droplet PCR (ddPCR, Bio-Rad, USA) (Figure 3B); the tumor was HER2-negative by immunostains. Plasma cell-free DNA (cfDNA) collected during ALK-TKI treatment was retrospectively analyzed with ddPCR and HER2-amplified circulating DNA was identified 2 months before clinical disease progression (Figure 3C). HER2 FISH analysis was then retrospectively performed on the primary tumor samples revealing a limited clone with HER2 amplification (Figure 2V,W,X). It was not possible to define the ALK status of these HER2-amplified cells given their small number and negative HER2 immunostains.
After liver biopsy and discontinuation of alectinib in January 2019, the clinical conditions of the patient rapidly worsened. A treatment with carboplatin-pemetrexed was not feasibly due to the poor performance status. Lorlatinib was started in February 2019 with a temporary clinical improvement (Figure 1A). The patient died in March 2019.
Discussion
We described a case of an ALK-positive NSCLC patient who developed clinical resistance to ALK-TKI therapy due to the emergence of a HER2-amplified clone. The primary tumor had heterogeneous histology, non-homogeneous ALK immunoreactivity and it was negative for ALK gene rearrangements by FISH. It harbored a minor HER2-amplified clone selected by ALK-TKI therapy to become the dominant component upon disease progression.
Most studies consistently report that ALK status, as assessed by IHC and FISH, is stable in primary NSCLC and its metastasis (9-11). However, at the cellular level, there is evidence of a significant intra-tumor heterogeneity of ALK expression that may underlie biological and clinical diversity (12). In the present case, the primary tumor was characterized by zonal variation in ALK immunostaining that in part segregated with different cell types, and it showed discordance between ALK IHC and FISH status in all samples. IHC and FISH discordance can occur, nonetheless, IHC positive/FISH negative NSCLCs have a high probability of response to ALK-inhibitors (13), as confirmed in present case. The patient, in fact, underwent first- and second-line ALK-TKI therapy with clinical benefit but, ultimately, she progressed. Biopsy of a liver metastasis showed an ALK-negative, histologically monomorphous tumor with diffuse HER2 gene amplification, as assessed by NGS sequencing and FISH. The emergence of this HER2-amplified clone could be traced 2 months earlier than the development of clinical resistance by the analysis of circulating tumor DNA with ddPCR. When retrospectively studied by FISH, both primary lung nodules and lymph node metastasis showed rare HER2-amplified cells (Figure 2V,W,X). Based on all these evidences, we conclude that the ALK-negative/HER2-amplified disease observed at progression derived from the selective pressure exerted by ALK-TKI therapy on a heterogeneous tumor harboring a primary resistant minor clone. The persistence and emergence of the resistant clone under sequential crizotinib and alectinib treatments suggests that HER2 amplification might cause resistance to both drugs.
To the best of our knowledge, this is the first report of HER2 gene amplification as a resistance mechanism to ALK-TKIs, both first- and second-generation. HER2 is known to cause resistance in EGFR-mutated patients treated with different generation of EGFR-TKIs (14,15). There is also limited evidence that it might be involved in resistance of ALK-positive NSCLC, evidence deriving from a preclinical study (16) and the analysis of a single case with several concomitant oncogenic drivers (17). Future treatment strategies of ALK-addicted NSCLC might benefit from a combined inhibition of both ALK and HER2 to minimize the development of resistance.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.04.03). MT serves as an unpaid editorial board member of Translational Lung Cancer Research from Dec 2019 to Nov 2021. MT reports grants and personal fees from Astra-Zeneca, personal fees from Pfizer, personal fees from Eli-Lilly, personal fees from BMS, personal fees from Novartis, personal fees from Roche, personal fees from MSD, grants and personal fees from Boehringer Ingelheim, personal fees from Otsuka, personal fees from Takeda, personal fees from Pierre Fabre, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Planchard D, Popat S, Kerr K, et al. Correction to: "Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up Ann Oncol 2019;30:863-70. [Crossref] [PubMed]
- Facchinetti F, Tiseo M, Di Maio M, et al. Tackling ALK in non-small cell lung cancer: the role of novel inhibitors. Transl Lung Cancer Res 2016;5:301-21. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Facchinetti F, Tiseo M. Digging into lorlatinib resistance in ALK-positive lung cancer: an editorial. Chin Clin Oncol 2019;8:S2. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Jamme P, Descarpentries C, Gervais R, et al. Relevance of detection of mechanisms of resistance to ALK inhibitors in ALK-rearranged NSCLC in routine practice. Clin Lung Cancer 2019;20:297-304.e1. [Crossref] [PubMed]
- Katayama R. Drug resistance in anaplastic lymphoma kinase-rearranged lung cancer. Cancer Sci 2018;109:572-80. [Crossref] [PubMed]
- Ma W, Guo L, Shan L, et al. Homogeneity and high concordance of ALK translocation in primary lung adenocarcinoma and paired lymph node metastasis. Sci Rep 2017;7:10961. [Crossref] [PubMed]
- Gao Q, Ma H, Wang B, et al. Comparison of ALK status between primary and corresponding lymph node metastatic tumors in lung cancer patients. Oncotarget 2017;8:108840-7. [Crossref] [PubMed]
- Zhu YC, Deng YT, Wang WX, et al. Clonally-related primary ALK rearranged adenocarcinoma and associated metastatic lesions. Thorac Cancer 2018;9:881-4. [Crossref] [PubMed]
- Cai W, Lin D, Wu C, et al. Intratumoral heterogeneity of ALK-rearranged and ALK/EGFR coaltered lung adenocarcinoma. J Clin Oncol 2015;33:3701-9. [Crossref] [PubMed]
- Marchetti A, Di Lorito A, Pace MV, et al. ALK protein analysis by IHC staining after recent regulatory changes: a comparison of two widely used approaches, revision of the literature, and a new testing algorithm. J Thorac Oncol 2016;11:487-95. [Crossref] [PubMed]
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29:i10-9. [Crossref] [PubMed]
- Minari R, Bordi P, Tiseo M. Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer: review on emerged mechanisms of resistance. Transl Lung Cancer Res 2016;5:695-708. [Crossref] [PubMed]
- Wilson FH, Johannessen CM, Piccioni F, et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 2015;27:397-408. [Crossref] [PubMed]
- Gu FF, Zhang Y, Liu YY, et al. Lung adenocarcinoma harboring concomitant SPTBN1-ALK fusion, c-Met overexpression, and HER-2 amplification with inherent resistance to crizotinib, chemotherapy, and radiotherapy. J Hematol Oncol 2016;9:66. [Crossref] [PubMed]


