Maximum standardized uptake value of primary tumor (SUVmax_PT) and horizontal range between two most distant PET-positive lymph nodes predict patient outcome in inoperable stage III NSCLC patients after chemoradiotherapy
Introduction
For patients with inoperable stage III non-small cell lung cancer (NSCLC), concurrent chemoradiotherapy (CRT) followed by consolidation therapy with PD-L1 inhibition is the current standard of care (1). Exact T- and N-stage definition is very important for the planning of multimodal treatment and patient prognosis. Also an assessment tool for functional impairment with Eastern cooperative oncology group (ECOG) is a metric to characterize patient prognosis (2). Previous prognostic scores have been proposed (3,4) and one of such scores was developed by our group from patient- and tumor-related factors (age, pack years, tumor-associated atelectasis and histology) and predicted prognosis in inoperable stage III NSCLC patients (5).
Whole body 18F-FDG-positron emission tomography (PET)/computed tomography (CT) is an established and validated diagnostic tool for initial staging in stage III NSCLC. FDG uptake is an indicator of tumor vitality that is usually measured using standardized uptake values (SUV), metabolic tumor volume (MTV) and total lesion glycolysis (TLG). FDG uptake intensity correlated with overall survival in different studies (6,7). Our previous data showed that an initial primary tumor (PT) MTV and its changes in the course and after completion of CRT has a prognostic impact in inoperable stage III NSCLC (8). Moreover, in a meta-analysis by Na et al., high pre-treatment SUVmax_PT was associated with poor outcome (9). Other studies have also shown a correlation between tumor histology and the SUV values in locally-advanced NSCLC (10,11).
The aim of present single-center analysis was to evaluate the impact of pre-treatment SUVmax_PT and range between two most distant PET-positive (SUV ≥2.5) lymph nodes in two directions (vertical and horizontal) on patient survival in a real-life inoperable stage III cohort. Hence, a new PET/CT score that combines these parameters was proposed and validated. To the best of our knowledge, no previous studies have combined multiple imaging parameters.
Methods
Patients
A total of 99 consecutive patients with locally advanced NSCLC stage IIIA–B (UICC 7th edition) between 2011 and 2016, treated with curative intent CRT were enrolled. All patients received initial staging including bronchoscopy with biopsy, contrast-enhanced CT or MRI of the brain. Histology yielded 49 adenocarcinomas (49.5%) and 50 non-adenocarcinomas (50.5%). Patients with distance metastasis on PET/CT and poor performance status (ECOG >1) were excluded from this analysis. This study was approved by the institutional review board.
CRT
All patients received platinum-based CRT (sequentially or concurrently). Various chemotherapy regimens were allowed. The majority of the cohort was treated with a combination of cisplatin 20 mg/m2 body surface area on days 1–4 and 50 mg/m2 oral vinorelbine on days 1, 8, 15 for 2 cycles.
A planning PET/CT was performed in the treatment position for improved target volume definition (tumor and affected lymph node regions). The description of target volume definition was previously published (5,12). Forty patients (40.4%) were treated with three-dimensional conformal radiotherapy, whereas 59 patients (59.6%) with intensity-modulated radiotherapy (IMRT). Treatment was delivered by a multi-energy linear accelerator. Image-guidance was performed with cone-beam CT several times a week.
18F-FDG-PET/CT
Emission scans were initiated 60–90 minutes after intravenous administration of 20 mg furosemide, 10–20 mg butylscopolamine and 18F-FDG. The examination was performed in the treatment position (patient’s arms overhead, wingstep) on carbon fiber couch at the same institution. After PET data collection, whole body CT scans were performed after intravenous injection of 100–120 mL iodized contrast. CT scans were also used to correct PET attenuation. The weight adapted to the body weight maximum SUV (SUVmax) of PT was determined. The SUV measurements were created using automated software in a 3D volume tool. (Hybrid Viewer 3D, Hermes Medical Solutions, Stockholm, Sweden).
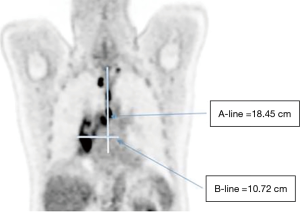
Lymph nodes with SUVmax ≥2.5 were indicated for malignant potential (13,14) and selected for the measurement of the two widest involved lymph nodes in selected CT layers with optimal imaging of lymph nodes in both directions. A-line was defined as the vertical distance of the 2 most distant involved pet-positive lymph nodes with SUVmax ≥2.5. B-line consisted of the horizontal distance of the 2 most distant affected lymph nodes (Figure 1). The measurement was performed by two experienced radiation oncologists and nuclear medicine specialists.
Statistics
Statistical analysis was performed using software of SPSS statistics 25 (IBM, New York, USA). The median follow-up achieved 17.2 (range: 2.2–92.1) months. Kaplan-Meier analyses were used to compare survival curves for the subgroups according to the SUVmax, vertical A- and horizontal B-lines. A receiver-operating characteristic (ROC) curve analysis was performed to define the best cut-off of SUVmax as well as vertical A- and horizontal B-lines in terms of overall survival. For the multivariate analysis, Cox regression model was used. All variables with P<0.1 (log-rank test) from previous analysis (age, pack years, tumor-associated atelectasis and histology) with this end point on univariate analysis were included in a multivariate cox regression analysis.
Results
Patient and treatment characteristics
A summary of patient and treatment characteristics is provided in Table 1. Of the 99 patients treated, 62 (62.6%) were men and 37 (37.4%) were women. The median age was 67 (range, 43–88) years. The median radiation dose was 60 (range, 45–70) Gy. The median interval between PET/CT and start of CRT was 12 (range, 0–67) days.
Full table
There was a significant positive relationship between: PT SUV and gross tumor volume, P<0.01; N stage and the sum of vertical A- and horizontal B-line, P<0.01; N stage and the horizontal B-line, P<0.01. In these patients no correlation could be found between SUVmax, vertical A- or horizontal B-line and histology.
SUVmax_PT
The mean SUVmax_PT was 11.6 (range, 0–41). The optimal cut-off value of pre-treatment SUVmax_PT was 8 based on the ROC analysis. The sensitivity, specificity and AUC using this cut-off values achieved 72%, 64% and 0.74 respectively. Among patients with SUVmax_PT ≥8, median OS was 19 months (95% CI: 15–23.2) vs. 40 months (95% CI: 9.8–70.2) in patients < SUVmax 8 (P<0.0001, log-rank test).
The median event-free survival (EFS) was 11.6 months (95% CI: 8.6–14.5) in the SUVmax ≥8 group and 16 months (95% CI: 13.9–17.8) in patients < SUVmax 8 (P<0.058, log-rank test).
Vertical (A-) and horizontal (B-) lines (involved lymph node distribution)
Vertikal A-line had no impact on overall survival at different cut-offs. Horizontal B-line cut-off ≥3.7 cm was significantly associated with worse survival compared to the rest of the treated cohort. The sensitivity, specificity and AUC using this cut-off values were 52%, 85% and 0.63 respectively. The median OS was 30 months when horizontal B-line <3.7 cm vs. 16.3 months when horizontal B-line ≥3.7 cm (P<0.01, log-rank test).
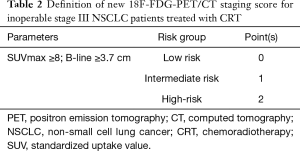
Evaluation of 18F-FDG PET-CT score
The score was developed on the basis of PET parameters correlated with OS on univariate analysis and included the SUVmax_PT and horizontal B-line. The patients were divided into 3 subgroups (Table 2). Low risk subgroup (0 points) included patients with SUVmax_PT <8 and horizontal B-line <3.7 (n=28, 28%). Forty-five patients (46%) had 1 point (intermediate risk) and 26 patients (26%) had 2 points (high-risk).
Full table
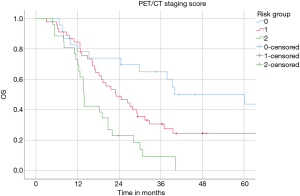
Median OS in the low, intermediate and high-risk subgroups was 40 (95% CI: 11–69), 23 (95% CI: 15–31) and 14 (95% CI: 13–14) months, respectively (P=0.0001, log-rank test) (Figure 2). The 2-year OS rates were 70% for low, 47% and 15% for intermediate and high-risk subgroups. The estimated 3-year OS rates were 52%, 24%, and 4% in the low, intermediate and high-risk patients, respectively.
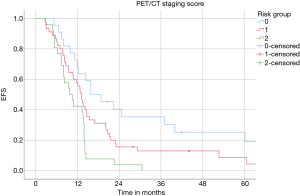
EFS was also significantly superior in the low (16 months) compared to intermediate (13 months) and high-risk patients (10 months) (P=0.002, log-rank test) (Figure 3). A 2-year EFS rate in the low risk was 32% vs. 0% in high-risk patients.
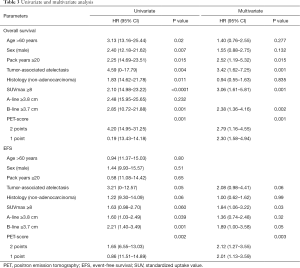
On multivariate Cox regression analysis, SUVmax_PT <8 and horizontal B-line <3.7 cm were significantly associated with OS [P=0.001, HR 3.06 (1.61–5.81) and P=0.002, HR 2.38 (1.36–4.16), respectively] and EFS [P=0.03, HR 1.91 (1.06–3.43) and P=0.04, HR 1.99 (1.02–3.87), respectively]. In addition, PET staging score was also an important variable and was a strong predictor of superior OS and EFS (P=0.001 and P=0.003, respectively) (Table 3).
Full table
18F-FDG-PET/CT score and histology
After stratification for histopathological tumor features, patients were divided into subgroups according to the PET-CT score. In the non-adenocarcinoma cohort, the analysis showed that patients with low risk had significantly longer overall survival than intermediate and high-risk (39 vs. 18 months, respectively, P=0.045). However, the subgroups showed only a trend for improved EFS (P=0.09). In contrast, OS and EFS differences among patients with adenocarcinoma were highly significant (P<0.0001 and P<0.001, respectively). The estimated median OS was not reached in the low-risk patients with adenocarcinoma. Median OS for the adenocarcinoma high-risk patients was only 12 (range, 10–14) months.
Discussion
Inoperable stage III NSCLC represents a heterogeneous disease based on total tumor volume and extension. These factors strongly influence multimodal treatment strategies and patient prognosis (15,16). Tumor extension has also a significant impact on the definition of treated (irradiated) volume and corresponding treatment toxicity (17,18).
An important role of 18F-FDG-PET/CT for initial staging and response characterization in locally-advanced NSCLC has been extensively described. Based on our data, initial PT-MV was defined as an important survival predictor (17). Patients with higher PT-MV (>63 cm3) before the start of multimodal treatment had significantly worse long-term outcome. Regarding the tumor response assessment after CRT, PT-MV reduction of at least 80% was necessary to significantly improve patient outcome (8). Furthermore, the last publication from the ACRIN 6668/RTOG 0235 trial showed that TLG at 175 and MTV at 35 cm3 were cutoff values for the definition of patient prognosis after completion of multimodal treatment (19). Furthermore, SUVmax_PT was also reported as a predictor of patient outcome in stage III NSCLC treated with CRT. Different SUVmax_PT cut-off values have been investigated (20). Kumasaka et al. showed that SUVmax_PT above the median of 9.7 significantly affects patient overall survival (HR 2.24, 95% CI: 1.29–3.88, P<0.01) (21). Analysis of 139 stage III NSCLC patients revealed an SUVmax_PT of 8.47 as a prognostic factor (22). The mean survival times of patients with SUVmax_PT >8.47 was only 15.9 vs. 32 months for the rest of the treated cohort.
On the basis of previous data and the present results, a PET/CT score was developed for patients with good performance status treated with CRT. This score includes SUVmax_PT as a metabolic parameter characterizing tumor vitality and growth as well as B-line (horizontal distribution) as an important characteristic of the metabolically active involved lymph node compartments. Both factors included in the present score were shown to have a significant impact on patient survival in the uni- and multivariate analyses.
According to the cumulative point number (0 to 2), the score was able to identify three different risk subgroups. Relevant survival differences could be demonstrated between patients with low, intermediate and high-risk, respectively. Especially long-term patient outcome, i.e., estimated 3-year OS rates, was significantly different between subgroups: from 52 in low to only 4% in the high-risk patients. Also, an estimated 2-year EFS rate of 32% in the low-risk versus 0% in high-risk patients. According to the presented score, low risk patients had the most favorable prognosis with a median survival of 40 months. In contrast high-risk patients demonstrated a dismal outcome in spite of good initial performance status and completed CRT. High-risk patients might mostly benefit from the individualization of the current multimodal approach. In this setting, intensification of combined treatment with chemo- and/or immunotherapy induction as well as the role of concurrent and sequential immune checkpoint inhibition require new studies.
The subgroup analysis demonstrated the special value of the PET-CT score for patients with adenocarcinoma. According to our score, the 2-year OS rate in low risk patients was 70% vs. only 8% in high-risk patients. As a result, this PET-CT score reliably identifies adenocarcinoma patients with a very poor outcome after CRT.
Important limitations of the present analysis are its retrospective and single-center design. Therefore, an independent validation in an external cohort is ongoing. In addition, PET emission scans were initiated 60 to 90 minutes after tracer injection. It is well known that SUV values increase over time after injection, so the involved nodes may be influenced depending on the time of scanning. False positive lymph nodes may explain the fact that in the subgroup analysis, the scoring system performed worse in SCC patients.
Conclusions
A PET/CT score for inoperable stage III NSCLC treated with CRT has been developed to estimate patient prognosis. The score includes two parameters: SUVmax_PT and horizontal B-line, characterizing PT vitality and growth as well as metabolically active involved lymph node compartments. This score was shown to be an independent prognostic factor in a single-center cohort and might be considered together with patient and treatment related factors for further optimization of multimodal therapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.04.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This analysis is in compliance with the principles of the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the Ludwig Maximilian University of Munich (No. 17-230). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Käsmann L, Taugner J, Eze C, et al. Performance status and its changes predict outcome for patients with inoperable stage III NSCLC undergoing multimodal treatment. Anticancer Res 2019;39:5077-81. [Crossref] [PubMed]
- Prelaj A, Ferrara R, Rebuzzi SE, et al. EPSILoN: A prognostic score for immunotherapy in advanced non-small-cell lung cancer: a validation cohort. Cancers (Basel) 2019. [Crossref] [PubMed]
- Gong J, Xu L, Li Z, et al. A Clinical prognostic score to predict survival of advanced or metastatic non-small cell lung cancer (NSCLC) patients receiving first-line chemotherapy: a retrospective analysis. Med Sci Monit 2018;24:8264-71. [Crossref] [PubMed]
- Taugner J, Käsmann L, Eze C, et al. Survival score to characterize prognosis in inoperable stage III NSCLC after chemoradiotherapy. Transl Lung Cancer Res 2019;8:593-604. [Crossref] [PubMed]
- Higashi K, Ueda Y, Arisaka Y, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 2002;43:39-45. [PubMed]
- Zhang C, Liao C, Penney BC, et al. Relationship between overall survival of patients with non-small cell lung cancer and whole-body metabolic tumor burden seen on postsurgical fluorodeoxyglucose PET images. Radiology 2015;275:862-9. [Crossref] [PubMed]
- Roengvoraphoj O, Eze C, Wijaya C, et al. How much primary tumor metabolic volume reduction is required to improve outcome in stage III NSCLC after chemoradiotherapy? A single-centre experience. Eur J Nucl Med Mol Imaging 2018;45:2103-9. [Crossref] [PubMed]
- Na F, Wang J, Li C, et al. Primary tumor standardized uptake value measured on F18-Fluorodeoxyglucose positron emission tomography is of prediction value for survival and local control in non-small-cell lung cancer receiving radiotherapy: meta-analysis. J Thorac Oncol 2014;9:834-42. [Crossref] [PubMed]
- Bashir U, Foot O, Wise O, et al. Investigating the histopathologic correlates of 18F-FDG PET heterogeneity in non-small-cell lung cancer. Nucl Med Commun 2018;39:1197-206. [Crossref] [PubMed]
- Kim E, Wu HG, Keam B, et al. Significance of 18F-FDG PET parameters according to histologic subtype in the treatment outcome of stage III non-small-cell lung cancer undergoing definitive concurrent chemoradiotherapy. Clin Lung Cancer 2019;20:e9-23. [Crossref] [PubMed]
- Nestle U, De Ruysscher D, Ricardi U, et al. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother Oncol 2018;127:1-5. [Crossref] [PubMed]
- Beggs AD, Hain SF, Curran KM, et al. FDG-PET as a "metabolic biopsy" tool in non-lung lesions with indeterminate biopsy. Eur J Nucl Med Mol Imaging 2002;29:542-6. [Crossref] [PubMed]
- Antoch G, Vogt FM, Freudenberg LS, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA 2003;290:3199-206. [Crossref] [PubMed]
- Käsmann L, Niyazi M, Blanck O, et al. Predictive and prognostic value of tumor volume and its changes during radical radiotherapy of stage III non-small cell lung cancer: a systematic review. Strahlenther Onkol 2018;194:79-90. [Crossref] [PubMed]
- Turgeon GA, Iravani A, Akhurst T, et al. What 18F-FDG PET response-assessment method best predicts survival after curative-intent chemoradiation in non-small cell lung cancer: EORTC, PERCIST, Peter Mac Criteria, or Deauville Criteria? J Nucl Med 2019;60:328-34. [Crossref] [PubMed]
- Roengvoraphoj O, Wijaya C, Eze C, et al. Analysis of primary tumor metabolic volume during chemoradiotherapy in locally advanced non-small cell lung cancer. Strahlenther Onkol 2018;194:107-15. [Crossref] [PubMed]
- Yue J, McKeever M, Sio TT, et al. Association of lung fluorodeoxyglucose uptake with radiation pneumonitis after concurrent chemoradiation for non-small cell lung cancer. Clin Transl Radiat Oncol 2017;4:1-7. [Crossref] [PubMed]
- Salavati A, Duan F, Snyder BS, et al. Optimal FDG PET/CT volumetric parameters for risk stratification in patients with locally advanced non-small cell lung cancer: results from the ACRIN 6668/RTOG 0235 trial. Eur J Nucl Med Mol Imaging 2017;44:1969-83. [Crossref] [PubMed]
- Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6-12. [Crossref] [PubMed]
- Kumasaka S, Nakajima T, Arisaka Y, et al. Prognostic value of metabolic tumor volume of pretreatment 18F-FAMT PET/CT in non-small cell lung Cancer. BMC Med Imaging 2018;18:46. [Crossref] [PubMed]
- Houdu B, Lasnon C, Licaj I, et al. Why harmonization is needed when using FDG PET/CT as a prognosticator: demonstration with EARL-compliant SUV as an independent prognostic factor in lung cancer. Eur J Nucl Med Mol Imaging 2019;46:421-8. [Crossref] [PubMed]