The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention
Introduction
Two years ago the 100-year anniversary of lung cancer was “celebrated”. 100 years after the first description of 374 cases of lung cancer (1) as high as 1.6 million new cases are diagnosed yearly (2,3). In recent years the clinical and biological patterns of lung cancer have been changed and are varying continuously. The increasing incidence of adenocarcinoma, decrease in the proportion of small cell lung cancer and new observations on lung cancer in nonsmokers are the most striking features of this change and remain a challenge for the progress in thoracic oncology. However, an unchanging fact is that lung cancer is the main oncological problem worldwide and it is a leading cause of cancer death among patients with malignancy. The resection rate that is a treatment of choice in early—stage non-small cell types—is as low as 25-30% (3,4). In the advanced stages of non-small cell lung carcinoma (NSCLC) other therapies are used with some effectiveness: chemotherapy, radiotherapy and biological treatment. Recently it became important to perform appropriate cancer molecular characterization and to select patients individually to a treatment strategy with thorough analysis of the histological type, molecular pattern and evaluation of predictive factors. In practice this molecular characterization is performed by analysis of activating mutations of epidermal growth factor receptor (EGFR) (in clinical practice in exons 19 and 21) and detection of an anaplastic lymphoma kinase (ALK) rearrangement (5). For tumors with activating EGFR mutations, first-line treatment is indicated with an EGFR tyrosine kinase inhibitor (EGFR-TKI, such as gefitinib, erlotinib, and afatinib). Anti-EGFR antibody- cetuximab is accepted in some countries as a biological therapy. The treatment with crizotinib is advised for ALK-positive lung cancer (5-7). However, the prevalence of an EGFR mutation in adenocarcinoma of European patients is close to 10%, while in Asian and Japanese patients is up to 30-50% (8). More lung cancer prognostic markers are being published, but without promising effectiveness in practice (5).
Among NSCLC subtypes adenocarcinoma is the most heterogeneous tumor, with known aggressiveness of certain subtypes (i.e., solid tumor with mucus production), and response to anti-EGFR targeted therapy in tumours harbouring EGFR mutations (9,10). This direction of targeted therapy has brought some good results, but only in the appropriate selected patients groups (5). Only a relatively small proportion of patients in our country harbor EGFR mutations so only small numbers of patients benefit from currently available targeted therapies (11). The current therapeutic approach develops in another direction—with taking into account an advantage of the recognition of the immune response in solid tumors. The goal of such new therapies is to support the host’s own anticancer immune response. Here a description of the immune alterations in the course of NSCLC with possible implications for therapy is presented.
Background to the considerations
The morbidity due to lung cancer is strongly correlated to age with the greatest risk in the oldest patients groups of both sexes. Age distribution at lung cancer diagnosis is estimated at approximately 6% in patients below 50 years of age, 29% in patients of 60-69 years old, and 44% in patients over 70 years of age (3). In this context the role of immune system senescence has to be revealed. The following alterations characterize an immune-aging (inflamm-aging): shortening of telomeres, histone acetylation and reduction of antiaging molecules such as histone deacetylases and sirtuins, apoptosis, increased concentration of proinflammatory cytokine- IL-6, and Th2 polarization (12). These disorders are inhibitors of anti-cancer immune response in the course of lung cancer. Immuno-senesce enhances the failure of anti-cancer response.
Cigarette smoking is the main risk factor for lung cancer (2,3). The influence of tobacco smoke on lung homeostasis is complex with a predominant feature being suppression of the immune system (13,14). We have previously reported the noxious influence of tobacco smoke on lung immune status (15-17). Apart from tobacco smoke, many other environmental agents permanently affect the lung milieu: dust, allergens and microbes, with resulting oxidative stress and hypoxia. These factors are capable of causing serious modification of lung immune status. For better understanding of the nature of immune disturbances, the continuous process of self- and down-regulation of the function of immune cells cannot be neglected.
The lung immune system has multiple parts: it consists not only of large numbers of immune cells with a complex cytokine network, but also of structural elements of different function, i.e., epithelial, endothelial and mesenchymal cells. In normal conditions an integration of these elements is fixed and the proportion of immune cells rests within a normal range. In my opinion a valuable way for evaluation of the lung immune status, in steady state and during disease, is bronchoalveolar lavage (BAL) fluid examination. BAL analysis is a low-invasive method and the BAL components reflect the local immune response in a large part of the lung. In clinical practice the main indication for BAL analysis is a diagnosis of diffuse parenchymal lung disorders, interstitial lung diseases and infections. In lung cancer the role of BAL in peripheral tumor diagnosis has also been documented (18). This is a quantitative method, already well standardized (19-21). For the lavage, 200 mL of saline is used; the total cell count and differential cell count are determined in the recovered fluid. The referenced BAL pattern in nonsmokers contains total cell count of about 10 million cells, cell viability is more than 90%, the percentage of macrophages >80%, lymphocytes <15%, neutrophils <5%, eosinophils <1% (19,21,22). The effectiveness of BAL in the evaluation of lung immune status in the course of lung cancer has been described in our earlier works (17,23-25). The elements of the immune response in lung cancer patients may serve as biomarkers and predictive factors in regards to immunotherapy, applied in clinical practice. BAL may be performed during bronchofiberoscopy, which is an inherent step in the diagnostic procedure. It should be mentioned that knowledge of defense mechanisms in lung cancer is rather limited to data obtained from peripheral blood samples, reflecting the systemic immune response. As concerns local immune response evaluation it is usually performed by the examination of resected tumors. Since the resection rate of NSCLC does not exceed 30% and small cell types are not-resectable per se, in the majority of lung cancer cases the local immune status cannot be studied. From this perspective the BAL analysis is important as it can be performed at any time of the disease, including advanced lung cancer stages.
Is the histologic type of NSCLC important? Today’s classification of NSCLC recommends clear distinction of squamous cell type and adenocarcinoma, which is important in guiding to current treatment with new methods of targeted therapy. In this context it is often important to detect thyroid transcription factor (TTF-1) positive cancer cells as an indicator of glandular differentiation in those cases where it cannot be seen morphologically. TTF-1 is essential for morphogenesis and differentiation of the lungs and is a marker of lung adenocarcinoma. In some studies TTF1 expressing tumors were suggested to be associated with longer survival (26,27). However, the heterogeneity of lung cancer occurs with mixed types entity. Moreover quite often the non-otherwise specified (NOS) type is being diagnosed. In these cases detection of TTF1 positive cells is suggestive for adenocarcinomatous differentiation. In the daily practice we observed that the EGFR mutation initially restricted to adenocarcinoma is present also in the squamous cell type. Perhaps in the future the molecular classification of NSCLC may be more relevant than the histological one.
Last but not least, cancer stem cells (CSCs) are a new potential target for solid tumor therapy, including a lung cancer therapy (28-30). The phenotype of CSCs is currently widely investigated, the marker CD133/EPCAM being suggested (31,32).
Cytotoxic attack
Lymphocytes, macrophages and granulocytes are involved in the anti-cancer battle. The niche of lymphocytes is known as tumor infiltrating lymphocytes (TIL) (33,34), of macrophages—the tumor associated macrophages (TAM) (35,36), of neutrophils—the tumor associated neutrophils (TAN) (37), and of eosinophils—the tumor associated tissue eosinophilia (TATE) (38). The main cell population with activity in anti-cancer immune response is the population of cytotoxic T lymphocytes (CTLs) (39). The CTLs population is represented by CD8+ lymphocytes, CD4+ lymphocytes, natural killer cells (NK), natural killer T cells (NKT) and lymphocytes B (40,41). Cancer cells are killed by induction of apoptosis by cytolytic reaction or membrane-receptor induction of programmed death. The successful cytotoxic attack needs an effective antigen presentation by tumor cells and antigen presenting cells (APC). This is achieved mainly by macrophages and dendritic cells (DCs) (42). The latter migrate to lymph nodes after contact with cancer antigens and activate effector cells by presenting the antigen. A crucial role in APC-lymphocyte signal transmission is played by co-stimulating molecules on APC and related receptors on lymphocyte (Figure 1) (39,43). As cytotoxic CD8+ lymphocytes and CD4+ cells are “soldiers” of the CTL army, the signal pathway B7-CD28 is widely investigated (39). The blockage of APC-CTL action is observed in malignancy and provides the CTLs inactivation.
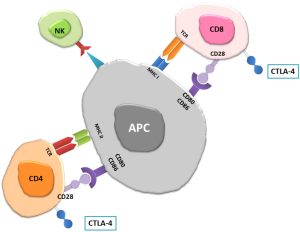
Impaired function of the immune system—the mechanisms of immune tolerance
It is well documented that anti-cancer defense is ineffective in clinically detectable cancers and that the greater is the size of a solid tumor mass, the less effective anti-cancer response is observed (44). Lung cancer cells hide against cytotoxic attack by low antigen presentation and low co-stimulatory molecule expression. Moreover, the lung cancer antigens are unstable and badly defined as a result of multiple genetic and epigenetic alterations during oncogenesis (45). Altogether, it leads to a passive cancer cells escape from immunosurveillance. On the other hand, many other elements of this escape relate to active regulation and suppression of the immune anti-cancer response.
There are many mechanisms of CTL inhibition (Figure 2). An interaction of programmed death receptors on lymphocytes with their ligands on tumor cells leads to apoptosis of lymphocytes. Recently it was revealed that the expression of the programmed death-1 (PD-1) molecule on T cells plays an important role in the context of cytotoxic effect inhibition (46). PD-1 is present on T helper, T cytotoxic, T regulatory cells, B lymphocytes and NK cells. Tumor cells express high levels of PD-1 ligands: B7-H1 (PD-L1) (CD274) and PD-L2 (CD273, B7-DC). The PD-1-PD-L interaction has a strong immunosuppressive effect. It has been applied to therapy with the blockade of PD-1/PD-L pathway using a fully humanized PD-1 or PD-L1 antagonistic monoclonal antibodies shown to increase the number and functionality of tumor-specific T cells (39,47-49).
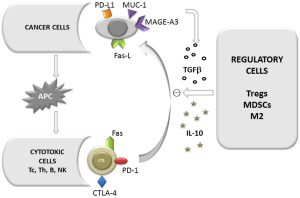
We have previously reported the increased expression of Fas receptor on lymphocytes in the course of lung cancer (50). An interaction of Fas-Fas ligand (Fas-L) causes the death of Fas bearing cells. Apart from an elevated proportion of Fas positive lymphocytes and the high expression of Fas receptor on CTLs, the expression of Fas-L on cancer cells is known to be markedly elevated. Alterations of the concentration of soluble forms sFas and sFasL which moderate apoptosis have been also found in NSCLC (51). Thus this receptor pathway plays an important role in the process of reduction of CTL number. To date no therapy targeting this mechanism is currently in clinical evaluation.
Another mechanism of impaired anticancer defense and hiding cancer cells from CTLs attack is a modification of co-stimulatory molecules on cancer cells and on APCs. T cells are the main cytotoxic population that recognizes target cells by interaction with APCs. B7 molecule (CD80/CD86) on the APC and the CD28 receptor on lymphocyte are necessary to activate the cytotoxic effect. However, B7 molecules are also capable of sending a suppressive signal by association with CTLA4 (Cytotoxic T cell antigen 4) (52-54). CTLA4 is a molecule capable of inhibiting the TCR signal on T cells having homology with the CD28-co-stimulatory molecule with strong affinity. CTLA4 leads to inhibition of cell cycle progression, decreased release of IL-2 and increased transforming growth factor beta (TGFβ) production by blocking CD28. By connection with forkhead box P3 (Foxp3), CTLA4 is constitutively expressed on regulatory T cells (Tregs) and promotes their regulatory function (55-57). There are two forms of CTLA-4 expression: on the cell surface after activation, and intracellularly as storage (58,59). Our study (data unpublished) showed a difference in the CTLA-4 expression on T cells deriving from peripheral blood (PB) of lung cancer patients and healthy subjects. The CTLA-4 surface expression in cancer patients was significantly higher, while the intracellular domain was decreased in PB of cancer patients compared to healthy subjects. Our results indicate the importance of cellular traffic of this molecule in malignancy.
Therapeutic approach I
PD-1 and CTLA-4 are considered the main checkpoint molecules for effective immunotherapy in solid tumors. PD-1 antagonists are presented by PD-1 or PD-L1 antibodies: nivolumab, lambrolizumab and pidilizumab. The results of recently ongoing trials with anti-PD-1 antibodies are promising, although the association with detection of PD-L1 on tumor cells before treatment is controversial (39,60).
The anti CTLA-4 IgG1 humanized antibody—ipilimumab binds to CTLA-4 and prevents the inhibition of CD28/B7 signaling. It leads to T cell activation and depletion of Tregs. Similarly to anti-PD-1 agents, the anti-CTLA-4 antibody has shown some benefits, particularly in combination with chemotherapy (48).
Recent studies confirm the importance of regulatory cells in the modification of immune response in malignancy. Regulatory T lymphocytes (Treg) are capable of inhibiting the function of CD4+ and CD8+ lymphocytes, dendritic cells and NK cells (55,61,62). Treg cells play an important role in the immune surveillance and tolerance. The source of natural Tregs (nTregs) is the thymus. The second source is a population activated peripherally (induced Tregs, iTregs). The suppressing cytokines: interleukin-10 (IL-10) and TGFβ are involved in the peripheral activation of Tregs (63). In the lung cancer milieu the concentrations of IL-10 and TGFβ is high, and these cytokines are secreted by cancer cells and immune cells stimulated by cancer (64). They constitute an active regulation of immune response by cancer through induction of Tregs. Tregs are identified by expression of the panel of antigens: a Foxp3, CD25, glucocorticoid-induced TNF-receptor (GITR) (CD357), lymphocyte-activation gene 3 (LAG3), cytotoxic T lymphocyte antigen-4 (CTLA4) and CD127. The Tregs are defined by expression of CD4, CD25, Foxp3 and low CD127 (65,66). Foxp3 is a transcription factor necessary to keep a proper Treg function. An increased expression of Foxp3 was found in the cancer cells and in TILs and the presence of Foxp3 in breast cancer as well as in lung cancer was a negative prognostic factor (65,67-71).
In addition to type Th1 and Th2 cells, the concurrent polarisation direction of T cells is Th17 differentiation. It is not so pronounced as Tregs, but regarded as significant in regulation of immune response in malignancy. These pluripotent cells are active in antimicrobial defense, albeit their proliferative and cytotoxic effect is low. Th17 cells are defined by production of IL-17A. Other cytokines play a role in Th17 differentiation, i.e., IL-6, IL-1β and IL-23. It is presumed that IL-6 inhibits Tregs development with stimulation of Th17 (72,73). This example of the plasticity of immune system is accomplished by known TGFβ function: TGFβ in low concentration induces Th17 differentiation, while in high concentrations induces Tregs Foxp3+ maturation (73). To our knowledge, there is no direct data on the anticancer effect of Th17. Until now some results indicate that the effect of Th17 is complex as the IL-17 action in cancer milieu is pleiotropic: suppressive and stimulating. The stimulating effect is related to proangiogenic role of IL17A (74-76).
Therapeutic approach II
The depletion of Tregs by anti-CD25 antibody was proven to be ineffective (77). More rational is putting efforts to change the polarisation of T cell by enzymatic and cytokine profile modification to achieve a re-polarization of Tregs to Th profile. Complex engineering by using the indoleamine 2,3-dioxygenase (IDO) inhibitor plus vaccine provided such a re-polarization (77).
Alveolar macrophages play an important role in lung cancer defense (35). In the solid tumors a population of TAM was widely investigated and their relation with cancer cells is complex. Generally, the function of TAM population is impaired, but their regulatory function in lung cancer immunity is postulated (78). Traditionally, macrophages were considered to be a uniform cell population, but recently have been divided to different phenotypes: M1, M2 and macrophages with regulatory properties (79-81). M1 macrophages as effector cells play an immunostimulating role by secretion of cytokines (IL-12 among others) and reveal phagocytic properties. M2 macrophages with their suppressive function are the main constituents of TAM population, promoting angiogenesis and wound healing (80). They release mainly IL-10. M1 and M2 are activated by different ways: M1 by LPS and IFNγ, while M2 by IL-4, IL-10, IL-13 and TGFβ. Such different polarization of macrophages is detected by diverse phenotype, i.e., M1 cells express mainly CD40, while M2 express CD163, as we have recently confirmed by immunocytochemistry staining (82). For regulatory macrophages no defined surface antigenicity was found, therefore identification is based on cytokine production (TGFβ and IL10). Further subtyping of the M2 population has been recently proposed on the basis of the inductors and mediators balance (83). The presence of M1 in cancer milieu is favorable (84), however M2 vastly predominate among TAM. The potential shift of M1-M2 was confirmed in our experiments by immunocytochemical staining (82).
Myeloid derived suppressor cells (MDSCs) originate as bone marrow derived hematopoietic cells and precursors of immune cells other than lymphocytes. An augmentation of circulating MDSCs in serious diseases and in malignancy has been documented (85). The MDSCs identification can be done by detection of antigens: CD11b, CD14, CD33, HLADR (85). The mediators secreting by cancer cells (i.e., GM-CSF, IL-6 and IL-1) are essential to MDSCs survival in the tumor microenvironment. MDSCs are able to inhibit T cells activation and DC differentiation, and to promote Tregs. Since arginine, cysteine and nitric oxide (NO) are necessary for a proper T cell activation and memory type differentiation, MDSCs inhibit immune response by competitive use of these substrates (86). MDSCs produce a number of radical species and suppressor cytokines, and by this way favour angiogenesis, vasculogenesis and metastases (39,87,88). The process of epithelial-mesenchymal transition (EMT) plays an important role in the context of MDSCs function and inflammatory cell migration. Until now some signaling pathways of cell to cell contact, cell polarity and cell- matrix modulation have been recognized however, the process is complex (89,90).
Therapeutic approach III
Efficacy of 5, 6-dimethylxanthenone-4-acetic acid (DMXAA, Vadimezan) for activation of the antitumor properties of TAM was described in an animal model by Fridlender et al. (91,92). Reduction of M2 and MDSCs function may be achieved by blocking the immunosuppressive enzymes and by reversing the hypoxia status in the tumor microenvironment (35,36,93). Nitroaspirin and sindelafil were found to be effective blockers of arginase and NO synthase, enhancing an effectiveness of anticancer vaccines (77). The anti IL-10 and anti-CD40 antibodies combined with chemotherapy were associated with the change of macrophage profile (94). Some unspecific substances are also capable of inhibiting MDSCs (39).
Cancer cells release many suppressor cytokines. In this context TGFβ is the best-recognized compound. An increasing concentration of TGFβ in cancer tissue and in the cancer cells culture as well as in the cancer milieu has been reported (15). The complex TGFβ function and role in tumor progression are presented in Figure 3. Several interleukins reveal similar immunosuppressive effect in the lung cancer environment, including IL-10 and IL-2. The latter induces CTLA4 and mediators: vascular endothelial growth factor (VEGF), prostaglandin E2, arginase, reactive oxygen species, sFas, sFasL (39,95).
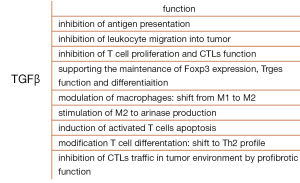
Regarding the complex role of TGFβ, it is unlikely that a use of a simple anti-TGFβ agent will be effective in cancer immunotherapy. Thus TGFβ is used only as an adjuvant in anti-cancer vaccines production and in combination with other therapies used for CTLs stimulation (39,96,97). Sometimes TGFβ promotes positive immune response and stimulates the CTLs, suggesting a pluripotent function of the cytokine (98). For example the interesting experimental study showed the different effect of TGFβ in relation to the time of tumor development: injection of anti-TGFβ agent before the injection of cancer cells resulted in inhibition of the active CTLs. Thus it may indicate a positive role of TGFβ in anticancer defense in the initial, pre-clinical stage of malignant disease (96).
Tumor antigens and vaccines production
There are two well-known lung cancer antigens that are used for vaccine production (99). The Melanoma Associated Antigen (MAGE-A3), absent on normal cells, is detected on NSCLC cells in about 35-50%, the majority being of squamous histological type (100). The presence of MAGE-A3 is associated with advanced stages of cancer. Some epitopes of this antigen are well recognized by HLA-I restricted lymphocytes Tc and these properties are used for vaccines production. Membrane associated glycoprotein (MUC-1) is associated with epithelial and glandular malignant tissue and is often overexpressed on cancer cells. A high MUC-1 expression is associated with lung cancer cell migration, resistance to apoptosis, and resistance to chemotherapeutic agents (101). The superficial domain of MUC-1 depending on the status of glycosylation is highly immunogenic; it makes possible the use of MUC-1 for T cell response stimulation (102). Recently another transmembrane glycoproteine-epithelial cell adhesion molecule (EpCAM) has been widely investigated in lung cancer; it was found that the detection of circulating lung cancer cells with EpCAM/MUC-1 overexpression was associated with poor prognosis after curative surgery (103).
There are numerous new neo-antigens recognized by genome sequencing of KRAS, EGFR, and ALK. The antigens and proteins encoded by these genes are present on lung cancer cells. The point mutations of these antigens make them immunogenic and useful for vaccine production (44,104).
The anti-cancer vaccines have been extensively investigated since the 1990s. The idea of vaccine production is to enhance antigen presentation by educated DCs. The vaccine formulation comprises the immunogenic tumor-associated antigens formed as peptides, recombinant proteins, gangliosides or whole tumor cells, which are combined with an adjuvant prior to potentiate the immune response (105). This immunoadjuvant is a viral vector, dendritic cell or liposome formulation. The examples of vaccines used in therapeutic approach in lung cancer are presented in the Table 1.
Full table
The results of recently conducted trials showed that anti-lung cancer vaccines failed to meet expectations with only some benefits in a selected group of patients (106). Therefore an effective direction in studies maybe individualization of immune treatment: the detection of cancer antigen before vaccination (MAGE-A3), enumeration of cytotoxic cells (anti-MUC-1 vaccine was shown to be effective in patients with normal number of activated NK cells) or individual production of dendritic cells with control of patient immune status (107). For evaluation of immunotherapy results the new criteria beyond RECIST WHO are needed and were recently described by Wolchok et al. on the basis of melanoma immunomodulating treatment (108). The immune-related response criteria (irRC) were introduced and the main consideration is that immunotherapy could be continued even in the case of radiological pattern of tumor progression.
The immune response in lung cancer is complex, hence the immunotherapy should be multivalent in combination with other therapeutic options. The most current promising direction is to combine immunotherapy with a conventional chemo- and radiotherapy. The rationale for such combination is manifold: by induction of immunogenic cell stress and cell death the cytotoxic agents are capable of enhancing tumor antigenicity, likewise radiotherapy can induce antigen expression and modulate antigenic repertoire (44). The regulatory/suppressor cells (Tregs, M2, MDSCs), an actively multiplied population, seem to be more susceptible to chemotherapy than the less numerous CTLs. Some cytotoxic agents have been shown to kill myeloid suppressor cells and inhibit FoxP3 expression, leading to reduction of the number of Tregs. Radiotherapy favors the release of proinflammatory cytokines, promotes antigen cross- presentation, recruits immune cells, supports DCs migration to lymph nodes and induces death cell receptors on tumor cells (39,44). The immunomodulatory properties of targeted therapy (e.g., cetuximab, crizotinib) have also been described (44,109,110). These observations are currently applied in clinical trials (111).
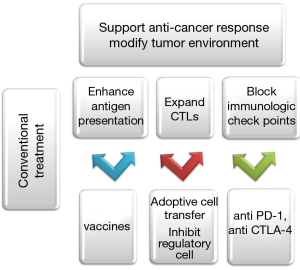
Figure 4 summarizes today’s goals of immunomodulating therapies in lung cancer. Almost every day delivers data on new therapeutical trials providing hopeful results in our battle against this tumor. Some limitation of the potential success of immunotherapy is due to the large number of advanced stages of NSCLC in time of the diagnosis and the fact that this kind of treatment is restricted to these stages in current clinical trials. However, the evidence of some benefit of complex treatment with immunotherapy as an additional arm with chemo- radiotherapy gives us hope for the future.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Adler IA. Classics in oncology: Primary malignant growths of the lung. CA Cancer J Clin 1980;30:295-301. [PubMed]
- Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S-29S.
- European Respiratory Society. eds. European Lung White Book. Washington, DC: European Respiratory Society, 2014.
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Thunnissen E, van der Oord K, den Bakker M. Prognostic and predictive biomarkers in lung cancer. A review. Virchows Arch 2014;464:347-58. [PubMed]
- Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer 2010;67:257-74. [PubMed]
- Thunnissen E, Bubendorf L, Dietel M, et al. EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch 2012;461:245-57. [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn 2013;15:415-53. [PubMed]
- Thunnissen E, Boers E, Heideman DA, et al. Correlation of immunohistochemical staining p63 and TTF-1 with EGFR and K-ras mutational spectrum and diagnostic reproducibility in non small cell lung carcinoma. Virchows Arch 2012;461:629-38. [PubMed]
- Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013;31:992-1001. [PubMed]
- Krawczyk P, Ramlau R, Chorostowska-Wynimko J, et al. The efficacy of EGFR gene mutation testing in various samples from non-small cell lung cancer patients: a multicenter retrospective study. J Cancer Res Clin Oncol 2015;141:61-8. [PubMed]
- Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244-54. [PubMed]
- Gonçalves RB, Coletta RD, Silvério KG, et al. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res 2011;60:409-24. [PubMed]
- Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol 2008;59:19-34. [PubMed]
- Domagała-Kulawik J, Hoser G, Safianowska A, et al. Elevated TGF-beta1 concentration in bronchoalveolar lavage fluid from patients with primary lung cancer. Arch Immunol Ther Exp (Warsz) 2006;54:143-7. [PubMed]
- Hoser G, Kawiak J, Domagała-Kulawik J, et al. Flow cytometric evaluation of lymphocyte subpopulations in BALF of healthy smokers and nonsmokers. Folia Histochem Cytobiol 1999;37:25-30. [PubMed]
- Hoser G, Domagała-Kulawik J, Droszcz P, et al. Lymphocyte subsets differences in smokers and nonsmokers with primary lung cancer: a flow cytometry analysis of bronchoalveolar lavage fluid cells. Med Sci Monit 2003;9:BR310-5. [PubMed]
- Poletti V, Poletti G, Murer B, et al. Bronchoalveolar lavage in malignancy. Semin Respir Crit Care Med 2007;28:534-45. [PubMed]
- Klech H, Pohl W. Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group. Eur Respir J 1989;2:561-85. [PubMed]
- Linder J, Rennard SI. eds. Bronchoalveolar Lavage. Chicago: The American Society for Clinical Pathology, 1992.
- Chciałowski A, Chorostowska-Wynimko J, Fal A, et al. Recommendation of the Polish Respiratory Society for bronchoalveolar lavage (BAL) sampling, processing and analysis methods. Pneumonol Alergol Pol 2011;79:75-89. [PubMed]
- Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. The BAL Cooperative Group Steering Committee. Am Rev Respir Dis 1990;141:S169-202. [PubMed]
- Domagała-Kulawik J, Hoser G, Droszcz P, et al. T-cell subtypes in bronchoalveolar lavage fluid and in peripheral blood from patients with primary lung cancer. Diagn Cytopathol 2001;25:208-13. [PubMed]
- Domagała-Kulawik J, Guzman J, Costabel U. Immune cells in bronchoalveolar lavage in peripheral lung cancer--analysis of 140 cases. Respiration 2003;70:43-8. [PubMed]
- Domagała-Kulawik J, Hoser G, Safianowska A, et al. Elevated TGF-beta1 concentration in bronchoalveolar lavage fluid from patients with primary lung cancer. Arch Immunol Ther Exp (Warsz) 2006;54:143-7. [PubMed]
- Bingle CD. Thyroid transcription factor-1. Int J Biochem Cell Biol 1997;29:1471-3. [PubMed]
- Solis LM, Behrens C, Raso MG, et al. Histologic patterns and molecular characteristics of lung adenocarcinoma associated with clinical outcome. Cancer 2012;118:2889-99. [PubMed]
- Wu X, Chen H, Wang X. Can lung cancer stem cells be targeted for therapies? Cancer Treat Rev 2012;38:580-8. [PubMed]
- Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823-35. [PubMed]
- Kim CF. Paving the road for lung stem cell biology: bronchioalveolar stem cells and other putative distal lung stem cells. Am J Physiol Lung Cell Mol Physiol 2007;293:L1092-8. [PubMed]
- Szypowska A, Stelmaszczyk-Emmel A, Demkow U, et al. Low frequency of regulatory T cells in the peripheral blood of children with type 1 diabetes diagnosed under the age of five. Arch Immunol Ther Exp (Warsz) 2012;60:307-13. [PubMed]
- Wang P, Gao Q, Suo Z, et al. Identification and characterization of cells with cancer stem cell properties in human primary lung cancer cell lines. PLoS One 2013;8:e57020. [PubMed]
- Yoshino I, Yano T, Murata M, et al. Phenotypes of lymphocytes infiltrating non-small cell lung cancer tissues and its variation with histological types of cancer. Lung Cancer 1993;10:13-9. [PubMed]
- Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008;14:5220-7. [PubMed]
- Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol 2012;167:195-205. [PubMed]
- Través PG, Luque A, Hortelano S. Macrophages, inflammation, and tumor suppressors: ARF, a new player in the game. Mediators Inflamm 2012;2012:568783.
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis 2012;33:949-55. [PubMed]
- Gatault S, Legrand F, Delbeke M, et al. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother 2012;61:1527-34. [PubMed]
- Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013;73:2381-8. [PubMed]
- Salagianni M, Baxevanis CN, Papamichail M, et al. New insights into the role of NK cells in cancer immunotherapy. Oncoimmunology 2012;1:205-7. [PubMed]
- Motohashi S, Okamoto Y, Yoshino I, et al. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol 2011;140:167-76. [PubMed]
- Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med 2005;172:530-51. [PubMed]
- Bright RK. Immunology of lung cancer. In: Pass HI, Mitchel IB, Johnson DH, et al. eds. Lung Cancer. Lippincott W&W, 2000:304-18.
- Tartour E, Zitvogel L. Lung cancer: potential targets for immunotherapy. Lancet Respir Med 2013;1:551-63. [PubMed]
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290:2149-58. [PubMed]
- Brahmer JR. PD-1-targeted immunotherapy: recent clinical findings. Clin Adv Hematol Oncol 2012;10:674-5. [PubMed]
- Dasanu CA, Sethi N, Ahmed N. Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert Opin Biol Ther 2012;12:923-37. [PubMed]
- Declerck S, Vansteenkiste J. Immunotherapy for lung cancer: ongoing clinical trials. Future Oncol 2014;10:91-105. [PubMed]
- Shih K, Arkenau HT, Infante JR. Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 2014;74:1993-2013. [PubMed]
- Hoser G, Wasilewska D, Domagała-Kulawik J. Expression of Fas receptor on peripheral blood lymphocytes from patients with non-small cell lung cancer. Folia Histochem Cytobiol 2004;42:249-52. [PubMed]
- Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother 2006;29:233-40. [PubMed]
- Erfani N, Mehrabadi SM, Ghayumi MA, et al. Increase of regulatory T cells in metastatic stage and CTLA-4 over expression in lymphocytes of patients with non-small cell lung cancer (NSCLC). Lung Cancer 2012;77:306-11. [PubMed]
- Erfani N, Khademi B, Haghshenas MR, et al. Intracellular CTLA4 and regulatory T cells in patients with laryngeal squamous cell carcinoma. Immunol Invest 2013;42:81-90. [PubMed]
- Mocellin S, Nitti D. CTLA-4 blockade and the renaissance of cancer immunotherapy. Biochim Biophys Acta 2013;1836:187-96.
- Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol 2004;16:89-98. [PubMed]
- Chambers CA, Kuhns MS, Egen JG, et al. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 2001;19:565-94. [PubMed]
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002;3:611-8. [PubMed]
- Iida T, Ohno H, Nakaseko C, et al. Regulation of cell surface expression of CTLA-4 by secretion of CTLA-4-containing lysosomes upon activation of CD4+ T cells. J Immunol 2000;165:5062-8. [PubMed]
- Wang XB, Zheng CY, Giscombe R, et al. Regulation of surface and intracellular expression of CTLA-4 on human peripheral T cells. Scand J Immunol 2001;54:453-8. [PubMed]
- Hall RD, Gray JE, Chiappori AA. Beyond the standard of care: a review of novel immunotherapy trials for the treatment of lung cancer. Cancer Control 2013;20:22-31. [PubMed]
- Becker C, Kubach J, Wijdenes J, et al. CD4-mediated functional activation of human CD4+CD25+ regulatory T cells. Eur J Immunol 2007;37:1217-23. [PubMed]
- D’Ambrosio D. Regulatory T cells: how do they find their space in the immunological arena? Semin Cancer Biol 2006;16:91-7. [PubMed]
- Sainz-Perez A, Lim A, Lemercier B, et al. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res 2012;72:3557-69. [PubMed]
- Orentas RJ, Kohler ME, Johnson BD. Suppression of anti-cancer immunity by regulatory T cells: back to the future. Semin Cancer Biol 2006;16:137-49. [PubMed]
- Shigematsu Y, Hanagiri T, Shiota H, et al. Immunosuppressive effect of regulatory T lymphocytes in lung cancer, with special reference to their effects on the induction of autologous tumor-specific cytotoxic T lymphocytes. Oncol Lett 2012;4:625-30. [PubMed]
- Simonetta F, Chiali A, Cordier C, et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol 2010;40:2528-38. [PubMed]
- Douglass S, Ali S, Meeson AP, et al. The role of FOXP3 in the development and metastatic spread of breast cancer. Cancer Metastasis Rev 2012;31:843-54. [PubMed]
- Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 2006;107:2866-72. [PubMed]
- Wolf AM, Wolf D, Steurer M, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003;9:606-12. [PubMed]
- Xue L, Lu HQ, He J, et al. Expression of FOXP3 in esophageal squamous cell carcinoma relating to the clinical data. Dis Esophagus 2010;23:340-6. [PubMed]
- Schneider T, Kimpfler S, Warth A, et al. Foxp3(+) regulatory T cells and natural killer cells distinctly infiltrate primary tumors and draining lymph nodes in pulmonary adenocarcinoma. J Thorac Oncol 2011;6:432-8. [PubMed]
- Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood 2013;121:2402-14. [PubMed]
- Romagnani S. Human Th17 cells. Arthritis Res Ther 2008;10:206. [PubMed]
- Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009;114:1141-9. [PubMed]
- Kryczek I, Wu K, Zhao E, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 2011;186:4388-95. [PubMed]
- Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol 2005;175:6177-89. [PubMed]
- Devaud C, John LB, Westwood JA, et al. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology 2013;2:e25961. [PubMed]
- Dabrowska M, Grubek-Jaworska H, Hoser G, et al. Effect of IFN-gamma stimulation on expression of intercellular adhesion molecule-1 (ICAM-1) on alveolar macrophages in patients with non-small cell lung cancer. J Interferon Cytokine Res 2006;26:190-5. [PubMed]
- Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. ScientificWorldJournal 2011;11:2391-402. [PubMed]
- Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549-55. [PubMed]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958-69. [PubMed]
- Osińska I, Wołosz D, Domagała-Kulawik J. Association between M1 and M2 macrophages in bronchoalveolar lavage fluid and tobacco smoking in patients with sarcoidosis. Pol Arch Med Wewn 2014;124:359-64. [PubMed]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014;6:13. [PubMed]
- Ma J, Liu L, Che G, et al. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010;10:112. [PubMed]
- Serafini P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly. Immunol Res 2013;57:172-84. [PubMed]
- Ino Y, Yamazaki-Itoh R, Oguro S, et al. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One 2013;8:e55146. [PubMed]
- Solito S, Marigo I, Pinton L, et al. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014;1319:47-65. [PubMed]
- Srivastava MK, Andersson Å, Zhu L, et al. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy 2012;4:291-304. [PubMed]
- Witz IP. Tumor-microenvironment interactions: the selectin-selectin ligand axis in tumor-endothelium cross talk. Cancer Treat Res 2006;130:125-40. [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54. [PubMed]
- Fridlender ZG, Jassar A, Mishalian I, et al. Using macrophage activation to augment immunotherapy of established tumours. Br J Cancer 2013;108:1288-97. [PubMed]
- Fridlender ZG, Albelda SM. Modifying tumor-associated macrophages: An important adjunct to immunotherapy. Oncoimmunology 2013;2:e26620. [PubMed]
- Doedens AL, Stockmann C, Rubinstein MP, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 2010;70:7465-75. [PubMed]
- Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612-6. [PubMed]
- Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother 2006;29:233-40. [PubMed]
- Quatromoni JG, Suzuki E, Okusanya O, et al. The timing of TGF-β inhibition affects the generation of antigen-specific CD8+ T cells. BMC Immunol 2013;14:30. [PubMed]
- Wallace A, Kapoor V, Sun J, et al. Transforming growth factor-beta receptor blockade augments the effectiveness of adoptive T-cell therapy of established solid cancers. Clin Cancer Res 2008;14:3966-74. [PubMed]
- Wahl SM. Transforming growth factor beta: the good, the bad, and the ugly. J Exp Med 1994;180:1587-90. [PubMed]
- Cuppens K, Vansteenkiste J. Vaccination therapy for non-small-cell lung cancer. Curr Opin Oncol 2014;26:165-70. [PubMed]
- Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 2013;31:2396-403. [PubMed]
- Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol 2012;13:e301-10. [PubMed]
- Roulois D, Grégoire M, Fonteneau JF. MUC1-specific cytotoxic T lymphocytes in cancer therapy: induction and challenge. Biomed Res Int 2013;2013:871936.
- Zhu WF, Li J, Yu LC, et al. Prognostic value of EpCAM/MUC1 mRNA-positive cells in non-small cell lung cancer patients. Tumour Biol 2014;35:1211-9. [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [PubMed]
- Mellstedt H, Vansteenkiste J, Thatcher N. Vaccines for the treatment of non-small cell lung cancer: investigational approaches and clinical experience. Lung Cancer 2011;73:11-7. [PubMed]
- Cuppens K, Vansteenkiste J. Vaccination therapy for non-small-cell lung cancer. Curr Opin Oncol 2014;26:165-70. [PubMed]
- Wojas-Krawczyk K, Krawczyk P, Buczkowski J, et al. Immunotherapy of lung adenocarcinoma patient with Peptide-pulsed dendritic cells: a case report. Arch Immunol Ther Exp (Warsz) 2012;60:69-77. [PubMed]
- Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [PubMed]
- Luedke E, Jaime-Ramirez AC, Bhave N, et al. Cetuximab therapy in head and neck cancer: immune modulation with interleukin-12 and other natural killer cell-activating cytokines. Surgery 2012;152:431-40. [PubMed]
- Luedke E, Jaime-Ramirez AC, Bhave N, et al. Monoclonal antibody therapy of pancreatic cancer with cetuximab: potential for immune modulation. J Immunother 2012;35:367-73. [PubMed]
- Declerck S, Vansteenkiste J. Immunotherapy for lung cancer: ongoing clinical trials. Future Oncol 2014;10:91-105. [PubMed]


