PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis
Introduction
Lung cancer remains the most lethal cancer worldwide, despite improvements in diagnostic and therapeutic techniques. Its incidence has not peaked in many parts of world, particularly in China, which has become a major public health challenge all the world (1). The prognosis for lung cancer patients is generally poor, with an overall 5-year survival rate of approximately 15%, and it has shown little improvement in recent decades (2,3). Several independent prognostic factors for survival have been identified: performance status (PS), disease stage, age, sex and amount of weight lost (4). Some of these factors are useful when choosing treatment options for an individual, principally disease stage and PS. However, the discriminant value of most potential prognostic biological markers is insufficient to predict the optimal therapeutic course for an individual (5,6).
The first duplication of programmed cell death-1 (PD-1) (B7-H1) was created based on its DNA sequence (7). PD-1, an immune checkpoint which is expressed on the surface of T, B and NK cells, is a surface-receptor member of the B7-CD28 superfamily (8). The key role of the PD-1 pathway plays in blunting the T cell immune function was confirmed for the first time in PD-1 knockout mice (9). Cells that express PD-1 evade T cell immunity via mechanisms such as exhaustion, apoptosis and anergy, and thereby defend tumor cells from cytolysis (10). Programmed cell death-ligand 1 (PD-L1), the major ligand for PD-1, is a cell surface protein in the B7 family which is found in tumor specimens from non-small cell lung cancer (NSCLC) patients (11). The association between PD-L1 overexpression and survival in lung cancer patients has been studied for several years. However, no consensus has been reached; conflicting results have been reported from different laboratories. We therefore carried out a meta-analysis of data from published studies to quantitatively review the effect of PD-L1 overexpression in tumor tissue on survival in patients with NSCLC.
Materials and methods
Search strategy and study selection
The electronic databases PubMed and China National Knowledge Infrastructure (CNKI) were searched for studies to include in our meta-analysis. An upper date limit of January 31, 2015 was applied; we used no lower date limit. Searches included the terms “Programmed cell death-ligand 1”, “PD-L1”, “B7-H1” and “prognosis”. We also reviewed the Cochrane Library for relevant articles. The references reported in the identified studies were also used to complete the search.
Studies eligible for inclusion in this meta-analysis met the following criteria: (I) measure PD-L1 expression in the primary lung cancer tissue with immunohistochemistry (IHC) or other methods; (II) provide information on survival (studies investigating response rates only were excluded); (III) have a follow up time exceeding 5 years; and (IV) when the same author reported results obtained from the same patient population in more than one publication, only the most recent report, or the most complete one, was included in the analysis. Two reviewers (PZ and ZZ) independently determined study eligibility. Disagreements were resolved by consensus.
Data extraction and quality assessment
The final articles included were assessed independently by two reviewers (PZ and ZZ). Data retrieved from the reports included first author, publication year, patient source, histology, disease stage, test method, PD-L1 positive and survival data (Table 1). If data from any of the above categories were not reported in the primary study, items were treated as “not applicable”. We did not contact the author of the primary study to request the information.
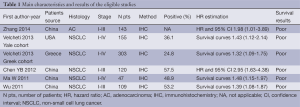
Full table
Statistical methods
For the quantitative aggregation of the survival results, hazard ratios (HR) and their 95% confidence intervals (CIs) were combined to give the effective value. When these statistical variables were not given explicitly in an article, they were calculated from available numerical data using methods reported by Parmar et al. (12).
Heterogeneity of the individual HRs was calculated with Chi-squared tests according to Peto’s method (13). Meanwhile, heterogeneity test with I2 statistic and Q statistic was performed. All the studies included were categorized by patient race, histology, disease stage. Individual meta-analysis was conducted in each subgroup. If HRs were found to have fine homogeneity, a fixed effect model was used for secondary analysis; if not, a random-effect model was used. In this meta-analysis, DerSimonian-Laird random effects analysis (14) was used to estimate the effect of PD-L1 overexpression on survival. By convention, an observed HR >1 implies worse survival for the group with PD-L1 overexpression. The impact of PD-L1 on survival was considered to be statistically significant if the 95% CI did not overlap with 1. Horizontal lines represent 95% CIs. Each box represents the HR point estimate, and its area is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with CI represented by its width. The unbroken vertical line is set at the null value (HR =1.0).
Evidence of publication bias was sought using the methods of Egger et al. (15) and of Begg et al. (16). Moreover, contour-enhanced funnel plot (17) was performed to aid in interpreting the funnel plot. If studies appear to be missing in areas of low statistical significance, then it is possible that the asymmetry is due to publication bias. If studies appear to be missing in areas of high statistical significance, then publication bias is a less likely cause of the funnel asymmetry. Intercept significance was determined by the t-test suggested by Egger (P<0.05 was considered representative of statistically significant publication bias). All calculations were performed using STATA version 11.0 (Stata Corporation, College Station, TX, USA).
Results
Study selection and characteristics
Five studies (18-22) published between 2011 and 2014 were eligible for this systematic review with meta-analysis. All reported the prognostic value of PD-L1 status for survival in NSCLC patients. The total number of patients included was 877 ranging from 47 to 303 patients per study (median 175). The major characteristics of the five eligible publications are reported in Table 1.
These publications followed several different patient cohorts. The NSCLC studies considered either all lung cancer subtypes (n=4) and adenocarcinomas (n=1). All five studies used IHC to evaluate PD-L1 expression in NSCLC. Among all the five studies evaluating PD-L1 expression in NSCLC, four studies (419 patients: 47.8%) were performed in Asian populations, and the remaining one study (458 patients: 52.2%) followed European or American patients. The proportion of patients exhibiting PD-L1 overexpression in individual studies ranged from 24.8% to 57.5%.
Meta-analysis
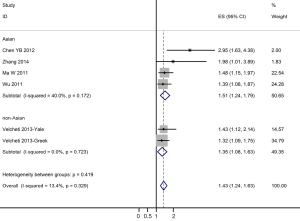
The results of the meta-analysis are reported in Figure 1. Overall, the combined HR for all eligible studies evaluated PD-L1 expression in NSCLC was 1.43 (95% CI: 1.24-1.63), indicating that PD-L1 overexpression was an indicator of poor prognosis for NSCLC patients. Meanwhile, no significant heterogeneity was detected among these studies (I2=13.4%, P=0.329). When grouped according to the geographic settings of individual studies, the combined HRs of Asian studies and non-Asian studies were 1.51 (95% CI: 1.24-1.7954) and 1.35 (95% CI: 1.08-1.63), respectively (Figure 1).
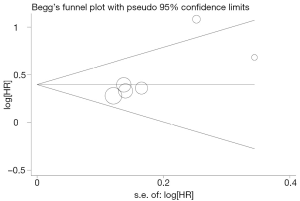
Publication bias
Begg’s funnel plot and Egger’s test were performed to assess the publication bias in the literature. All five eligible studies investigating NSCLC patients yielded a Begg’s test score of P=0.274 and an Egger’s test score of P=0.183, meanwhile according to the contour-enhanced funnel plot (Figure 2), the absence of publication bias was found in all five studies. These results suggest that there is no publication bias.
Discussion
PD-L1 expression leads to a negative antitumor immune response (23). PD-L1 is a ligand for PD-1, which is expressed on the surface of immune cells. By means of the PD-1/PD-L1 pathway, PD-L1 enables cancer cells to evade the host’s immune system via the apoptosis of T-cell clones, the inhibition of lymphocyte proliferation and T-cell cytokine secretion (24-26). CD8þT-cells play a major role in cellular responses, including in antitumor immune defense, while tumor-infiltrating lymphocytes (TILs) contribute to good clinical outcomes in many types of cancer (27). Fewer TILs have been found in PD-L1-positive regions compared with PD-L1-negative regions, which means that PD-L1 expression appears to have a negative effect on the host’s antitumor response (23).
In the present meta-analysis, we have combined five published studies including 877 patients with NSCLC to yield summary statistics that indicate that PD-L1 overexpression has a significant correlation with poor survival in NSCLC and adenocarcinoma patients. This correlation was observed in both Asian and non-Asian study populations.
Recently, several systematic reviews (28-36) with meta-analyses on other biological prognostic factors for NSCLC had been reported. P53, microvessel density, HER-2, Ki-67 and RAS might be poor prognostic factors for survival in NSCLC, however, Bcl-2 might be better prognostic factor for survival in NSCLC. In order to clarify the prognostic impact of other biological factors in lung cancer, our group has performed several systematic reviews of the literature with meta-analyses. We found that vascular endothelial growth factor (VEGF) (37), E-cadherin (38) and matrix metalloproteinase 2 (39) might be poor prognostic factor in NSCLC, COX-2 (40) might be poor prognostic factor for stage I NSCLC, the ground glass opacity (GGO) area (41) had a favorable prognostic value of overall survival and relapse-free survival in small lung adenocarcinoma.
However, there are limitations to our study. This meta-analysis was limited to articles published in English and Chinese and could not include studies that were not published due to negative or useless results. Another potential source of bias is related to the method of HR and 95% CI extrapolation. If these statistics were not reported by the authors, we calculated them from the data available in the article. If this was not possible, we extrapolated them from the survival curves, necessarily making assumptions about the censoring process. Data for multivariate survival analysis reported in the article were included in the present systematic review with meta-analysis; if these data were not available, data calculated from survival curves by univariate analysis were included. These results should be confirmed by an adequately designed prospective study. Furthermore, the exact value of PD-L1 overexpression status needs to be determined by appropriate multivariate analysis. Unfortunately, few prospectively designed prognostic studies concerning biomarkers have been reported; thus, our collection of many retrospective studies revealed more significance.
Publication bias (42) is a major concern for all forms of meta-analysis; positive results tend to be accepted by journals, while negative results are often rejected or not even submitted. The present analysis does not support publication bias; the obtained summary statistics likely approximate the actual average. However, it should be noted that our meta-analysis could not completely exclude biases. For example, the study was restricted to papers published in English and Chinese, which probably introduced bias.
In conclusion, PD-L1 overexpression is associated with a poor prognosis in patients with NSCLC in present meta-analysis. These results should be confirmed by an adequately designed prospective study.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [PubMed]
- Alberg AJ, Ford JG, Samet JM, et al. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:29S-55S.
- Paesmans M, Sculier JP, Libert P, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J Clin Oncol 1995;13:1221-30. [PubMed]
- Donnem T, Bremnes RM, Busund LT, et al. Gene expression assays as prognostic and predictive markers in early stage non-small cell lung cancer. J Thorac Dis 2012;4:212-3. [PubMed]
- Osarogiagbon RU. Predicting survival of patients with resectable non-small cell lung cancer: Beyond TNM. J Thorac Dis 2012;4:214-6. [PubMed]
- Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365-9. [PubMed]
- Creelan BC. Update on immune checkpoint inhibitors in lung cancer. Cancer Control 2014;21:80-9. [PubMed]
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 2002;169:5538-45. [PubMed]
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467-77. [PubMed]
- Shi L, Chen S, Yang L, et al. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol 2013;6:74. [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [PubMed]
- Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335-71. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Palmer TM, Peters JL, Sutton AJ, et al. Contour-enhanced funnel plots for meta-analysis. Stata J 2008;8:242-54.
- Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 2014;7:567-73. [PubMed]
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. [PubMed]
- Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 2012;98:751-5. [PubMed]
- Ma W, Luo DZ, Chen Y, et al. Expression and clinical significance of PD-L1 and PD-1 in non-small cell lung cancer. The Journal of Practical Medicine 2011;27:1551-4.
- Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011;28:682-8. [PubMed]
- Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094-100. [PubMed]
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer 2006;119:317-27. [PubMed]
- Tsushima F, Yao S, Shin T, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood 2007;110:180-5. [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [PubMed]
- Prado-Garcia H, Romero-Garcia S, Aguilar-Cazares D, et al. Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clin Dev Immunol 2012;2012:741741.
- Steels E, Paesmans M, Berghmans T, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J 2001;18:705-19. [PubMed]
- Meert AP, Paesmans M, Martin B, et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2002;87:694-701. [PubMed]
- Meert AP, Martin B, Paesmans M, et al. The role of HER-2/neu expression on the survival of patients with lung cancer: a systematic review of the literature. Br J Cancer 2003;89:959-65. [PubMed]
- Martin B, Paesmans M, Berghmans T, et al. Role of Bcl-2 as a prognostic factor for survival in lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2003;89:55-64. [PubMed]
- Martin B, Paesmans M, Mascaux C, et al. Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer 2004;91:2018-25. [PubMed]
- Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2005;92:131-9. [PubMed]
- Meert AP, Martin B, Delmotte P, et al. The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. Eur Respir J 2002;20:975-81. [PubMed]
- Nakamura H, Kawasaki N, Taguchi M, et al. Survival impact of epidermal growth factor receptor overexpression in patients with non-small cell lung cancer: a meta-analysis. Thorax 2006;61:140-5. [PubMed]
- Fan J, Wang L, Jiang GN, et al. The role of survivin on overall survival of non-small cell lung cancer, a meta-analysis of published literatures. Lung Cancer 2008;61:91-6. [PubMed]
- Zhan P, Wang J, Lv XJ, et al. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol 2009;4:1094-103. [PubMed]
- Wu Y, Liu HB, Ding M, et al. The impact of E-cadherin expression on non-small cell lung cancer survival: a meta-analysis. Mol Biol Rep 2012;39:9621-8. [PubMed]
- Qian Q, Wang Q, Zhan P, et al. The role of matrix metalloproteinase 2 on the survival of patients with non-small cell lung cancer: a systematic review with meta-analysis. Cancer Invest 2010;28:661-9. [PubMed]
- Zhan P, Qian Q, Yu LK. Prognostic value of COX-2 expression in patients with non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2013;5:40-7. [PubMed]
- Miao XH, Yao YW, Yuan DM, et al. Prognostic value of the ratio of ground glass opacity on computed tomography in small lung adenocarcinoma: A meta-analysis. J Thorac Dis 2012;4:265-71. [PubMed]
- Begg CB, Berlin JA. Publication bias: a problem in interpreting medical data. J R Stat Soc Series A 1988;151:419-63.


