Three-dimensional reconstruction/personalized three-dimensional printed model for thoracoscopic anatomical partial-lobectomy in stage I lung cancer: a retrospective study
Introduction
Three-dimensional (3D) printing was initially reported as early as the 1980s (1). This technology, which can print objects of any shape, is a revolutionary technological innovation. Now, this technology is of considerable significance to highly personalized medicine, which enables building accurate patient-specific 3D printed anatomic models that can be used for new surgical instrument development, physical measurements, diagnosis, surgical planning, and presentation to patients (2-6). Besides, such models are useful for educational purposes and physiologic simulations in resident physician training. 3D printing technology is also used in the department of radiation oncology (7). Its life-like features make it useful for the optimization and validation of image acquisition, processing and reconstruction for radiotherapy-related research (8). 3D printing technology realizes the leap-forward transformation from a digital 3D virtual image to a physical geometry model. Its research and achievements benefit the implementation and popularization of precise surgery.
With the renewal of surgical concepts and advancements of surgical skills, recent studies indicated that segmentectomy resection could be enough for clinical stage I lung cancer when it is appropriately selected (9-11). Anatomical partial-lobectomy (APL) is a concept of oncology therapeutics proposed by our center, which based on the anatomy of pulmonary segments and subsegments (12). This concept of surgical treatments is defined as anatomical sublobular resection, including single-segmentectomy, extended-segmentectomy, and combined-subsegmentectomy etc., but not non-anatomic procedure such as wedge resection. The procedure of APL entirely takes into account the individualized surgical differences of sub lobectomy in different patients based on tumor location. For multiple pulmonary nodules, APL may be a better option, especially for those located in different lobes. The difficulty of APL is more significant than that of lobectomy for the reason that the anatomy of lung segments, especially sub-segments, are more complicated. Vascular and bronchial variations of lung segments are common. For instance, variation pattern in right upper pulmonary veins alone was classified into four types, and each of these types was further divided into different subcategories according to Shimizu et al. (13). On the other hand, the vessels are thinner and more fragile so that more difficult to denude.
Detailed preoperative planning and simulations of anatomic sublobular resection using 3D technology would significantly contribute to safe operation. However, conventional 3D imaging is limited by a presentation on a flat screen and can hamper full visualization of complex pulmonary anatomy during operation. Compared with the virtual images of 3D reconstruction, the 3D printing model provides a more direct view instead of brain imagination for surgeons to identify the complicated and entangled vessels and bronchi before APL. The effectiveness of 3D reconstruction and 3D printing model in APL was described in our retrospective study. This study then evaluated the significance of personalized 3D printing model in the preoperative period for stage I lung cancer. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-571).
Methods
Patients
We retrospectively analyzed the records of 298 patients who underwent APL in our hospital from April 2017 to May 2019. The eligibility criteria were as follows: (I) clinical diagnosed for NSCLC and total size of the lesion ≤2 cm; (II) each patient received intensively segmentectomy according to the National Comprehensive Cancer Network (NCCN) guidelines for NSCLC (14), with or without 3D reconstruction before the operation, and some cases with 3D reconstruction were further provided with 3D printing models; (III) no limitation on age or gender; (IV) definitive preoperative diagnosis by chest computed tomography (CT), head magnetic resonance imaging (MRI), abdominal ultrasound (or CT), bone scan or positron emission tomography (PET) excluding distant metastasis, and routine assessment of cardiopulmonary function excluding surgery contraindications; (V) no neoadjuvant chemotherapy or radiotherapy treatment had been administered.
Patient characteristics and clinical outcomes were retrospectively reviewed from the prospectively collected database of our department. This study was approved by the ethics committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (approval number: 191137-1921). The requirement of patients’ informed consent was waived owing to the retrospective nature of the study.
Preoperative 3D image construction and 3D model printing
The preoperative CT data of each patient enrolled in 3D-reconstruction and 3D-model groups obtained from the imaging workstation in our institute. The digital imaging and communications in medicine (DICOM) data of thin-slice (0.625–1.25 mm) CT images were imported into Mimics 21.0 (developed by Materialise Nv Co., Materialise’s interactive medical image control system, Kingdom of Belgium) for 3D reconstruction of bronchi and blood vessels. The resection region and cutting plane were manually designed based on the location of the lesion in the 3D image. Ensure that the shortest distance from the resection margin of lung parenchyma to the edge of the tumor was more than 2 cm or max tumor size. In the 3D-model group, the ‘stereolithographic (STL)’ format derived from the reconstruction image was processed by Materialise 3-Matic Software (developed by Materialise Nv Co., Kingdom of Belgium), then the 3D model was printed.
Operative procedure
Patients were administered general anesthesia with double-lumen tracheal intubation, one-lung ventilation. The thoracoscopic APL was performed in all patients via a three-port approach.
The target bronchus and vascular structures were individually isolated. After the division of the target artery and bronchus, the “inflation-deflation method” was used to ascertain the cutting plane/intersegmental line. The extent of resection was decided by sufficient resection margin. The management of the cutting plane/intersegmental plane (electrocautery, staplers, or combined application of each method) was considered relevant with postoperative comorbidities (i.e., air leak, hemoptysis). The hilar and mediastinal lymph nodes should be sampled or systematically dissected if the rapid-freezing pathology of any nodes was malignant during the surgery. APL of simple segments was defined as resection of only 1 intersegmental plane, such as lingual segmentectomy, whereas APL of complex segments was defined as the resection of 2 or more intersegmental planes combined-subsegmentectomy.
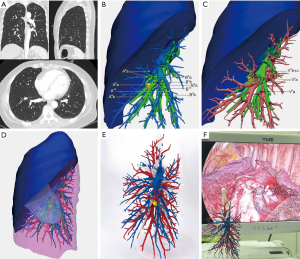
In the non-3D group (n=136), we used preoperative chest CT scans to ascertain the location of the nodules and the anatomy of pulmonary segmental vessels and bronchi. The procedure followed was similar to the 3D reconstruction (n=131) or 3D printing model group. In the 3D reconstruction group, virtual visualization of 3D images was used to confirm anatomical variation and preoperative planning. In the 3D printing model group (n=31), a personalized 3D printed model was placed in front of a thoracoscopic screen in the operating room so that the operator could observe the anatomy of segmental vessels and bronchi at any time, which give surgeons cross-reference to perform the surgery (Figure 1A,B,C,D,E,F). Video 1 shows the procedure of a typical anatomical partial lobectomy (LS8a + LS9a).
Postoperative follow-up of patients was scheduled for outpatient review after 1 month (X-ray or CT) and 3 months (CT).
Analyzing evaluation data
Clinical pathological data and perioperative indicators were collected. Perioperative indicators included operation time, intraoperative blood loss, postoperative drainage, chest tube duration, postoperative hospital stay, and complication. Surgeons rechecked the data of perioperative indicators. The development of postoperative complication in this study was defined as grade 2 or above for severe complications under the Clavien-Dindo classification system (15). Surgeons evaluated the satisfaction and utility of 3D models with a subjective questionnaire. Then, we discussed technical details and applications of the 3D technique, intending to provide some clinical recommendations, which may contribute to current practice and future study.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software (IBM Corp., Armonk, NY, USA). The measurement data was expressed by “mean ± standard deviation (±SD)”, and ANOVA analysis was used for comparison when groups are more than two. The number of cases (n) was used to express the counting data, and the χ2 test was used to compare the rate of counting data between groups (%). A P value of less than 0.05 was considered statistically significant.
Results
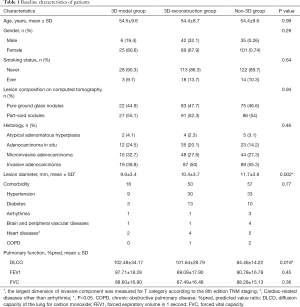
The characteristics of the patients who underwent thoracoscopic APL during this study period are summarized in Table 1. The 298 patients included 83 men (27.85%) and 215 women (72.15%), aging from 28 to 75 years (mean 54.4 y). One hundred eighty lesions (46.88%) were pure ground-glass nodules (pGGNs) and 204 lesions (53.13%) were part-solid nodules (PSNs). Almost all pulmonary nodules were detected by regular physical examination or CT screening, except 13 patients with apparent symptoms of cough or chest pain. Baseline data showed that the maximum CT diameter of pulmonary nodules in the 3D model group was significantly smaller than that in the 3D-reconstruction and non-3D group (P=0.002). All thoracoscopy procedures were completed without conversion to thoracotomy. According to the postoperative pathological results, the percentage of invasive adenocarcinoma (50.78%, 195/384) in all lesions were similar to non-invasive adenocarcinoma [adenocarcinoma in situ/ atypical adenomatous hyperplasia/ microinvasive adenocarcinoma (49.23%, 189/384)]. The percentage of invasive adenocarcinoma in PSNs was higher than that in pGGNs (69.61% versus 29.44%). No patients had lymphatic metastasis. Patients were examined by the followed-up chest CT scan three months after the operation and found no death or severe complications outside the hospital.
Full table
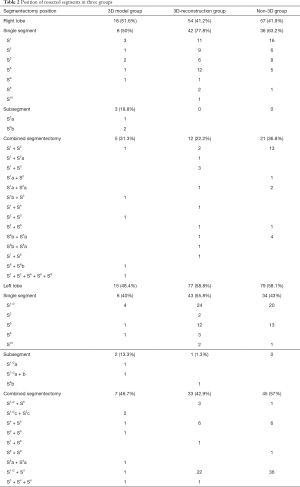
The types and positions of resected segments in the three groups are shown in Table 2. 116 cases (38.9%) were simple segmentectomy, 182 cases (61.07%) were complex segmentectomy. In the 3D model group, only 4 cases underwent APL with a simple segment. Table 3 showed that the proportion of complex segmentectomy in 3D model (87.1%) group was higher than that in 3D-reconstruction (60.3%) and non-3D (55.9%) group (P=0.006). In the non-3D group, 2 cases underwent extended wedge resection due to the insufficient resection margin distance, and 2 cases of left S1+2 (LS1+2) adjusted to LS1+2 + LS3 because of vascular variations discovered during surgery.
Full table

Full table
The intra-operative and postoperative characteristics of 298 patients undergoing APL were shown in Table 4. There were no 90-day operative mortalities. The pathological results of lymph nodes showed no metastasis. In the 3D-reconstruction and 3D model group, the intra-operation conditions were basically the same as the preoperative 3D images or models. All anomalous or uncommon bronchioles and vessels were accurately identified by 3D imaging. For complex segmentectomy, the operation time of the 3D model group (99.6±21.7 min) was shorter than that of 3D-reconstruction (116.1±30.7 min) (P=0.011) and non-3D group (125.1±23.6 min) (P<0.001). Meanwhile, the 3D-reconstruction group spent a shorter operation time when compared with the non-3D group (P=0.041). However, for simple segmentectomy, there was no significant difference among the three groups in operation time. Intraoperative bleeding in 3D model group (12.9±7.8 mL) was significantly lower than in 3D-reconstruction (20.9±12.2 mL) (P=0.001) and non-3D group (18.2±12.2 mL) (P=0.022). There were only four patients who occurred severe postoperative complication, three cases in the 3D-reconstruction group, and one in the non-3D group. The cases of complications were too few to be compared statistically. The postoperative drainage and hospital stay were similar among the three groups (all P>0.05).
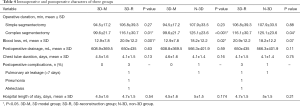
Full table
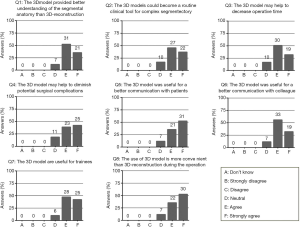
A total of 59 surgeons who had used 3D reconstruction imaging or 3D printed model completed the questionnaire. The results are shown in Figure 2. In total, 88% of the surgeons agreed that the 3D models provided a better understanding of the thoracic segmental anatomy than 3D-reconstruction and seven surgeons were neutral. After further inquiries, seven surgeons suggested that for experienced doctors, 3D reconstruction imaging is enough to understand the segmental anatomy. However, for beginners or less experienced doctors, 3D printed models might be better. Because of the economic cost, ten doctors (16.9%) were neutral about popularizing the 3D printed model as a routine tool for complex thoracic segmental resection. More than half of the surgeons (50.9%) strongly agreed that the 3D model was more convenient than 3D-reconstruction imaging during the operation. In total, 88% of the surgeons agreed that 3D models were useful for better communication with patients or other colleagues. Less substantially but still positively, 81.4% [48] of surgeons agreed or strongly agreed that the model might help to diminish potential surgical complications. Overall, surgeons scored their satisfaction with 3D models as 8.0 out of 10.
Discussion
In recent years, personalized 3D models showed increasing competitiveness in preoperative planning of complicated thoracic surgery such as anatomical sublobular resection (16,17). However, this technique has been reported only in very few papers, mainly focusing on case reports with lack rigorous statistical studies (18,19). Methodologically, the best evidence is obtained through clinical randomized controlled trials. However, not all patients are willing to choose to spend some money to have 3D printed models voluntarily, it is challenging to perform prospective controlled studies. Our study is a retrospective controlled study to compare 3D reconstruction imaging and 3D model, and confirms that personalized 3D printed models have unique advantages in APL. Due to the complexity and variability of segmental blood vessels and bronchi, APL is technically more complex than lobectomy, although it simplifies precise segmentectomy to some extent. In 116 cases of APL with simple segmentectomy, there was no significant difference in operation time in three groups. Therefore, the significance of reconstructing and printing models is more remarkable in the APL of complex segmentectomy.
In some cases of simple segmentectomy, impact of 3D printed models on surgical planning and intraoperative operation may not be significant. In these cases, extra value of 3D models may not compensate the time and cost involved in manufacturing process of the model. In this study, only 4 of the 31 patients in the 3D model group underwent APL of simple segmentectomy, which was much fewer than that in the other two groups. Therefore, for APL of complex lung segmentectomy, 3D reconstruction/personalized 3D printing model plays a decisive role.
The advantages of 3D reconstruction before segmentectomy can be concluded in two aspects. Firstly, preoperative reconstructed imaging can effectively identify pulmonary variant vessels and bronchi (13). Hagiwara et al. demonstrated the validity of preoperative 3D-CT imaging in assessing vascular branching patterns. In total, 97.8% of pulmonary artery branches can be precisely identified, and the incidence of comorbidities after an operation can be significantly reduced (20). The 3D imaging was also used to identify the intersegmental veins as boundary lines of the pulmonary segments. Secondly, 3D reconstruction imaging can accurately locate pulmonary nodules and target segments (21). Several studies have reported the feasibility of this technique in locating pulmonary nodules in deep parenchyma or adjacent segments boundary. In the study of Xue et al., 19% patients changed original surgical plans according to the simulating results. At the same time, some cases with inadequate resection margin distance were then undergone enlarged wedge resection in the non-3D imaging group (22). Similar conditions were also observed in our study. However, due to the possible bias of case selection, the result was not so apparent as previous studies. In fact, to design margin or intersegmental line before operation by using 3D reconstruction is a critical step in APL. Current researches are limited to preoperative planning using 3D reconstruction and simulation (23). There is no in-depth study on the guidance of intra-operation. In our study, a personalized 3D printed model provides a quick reference for the process of APL.
The 3D visualization imaging was limited by computer 2D display about the sense of space and distance, which was still not sufficient enough to meet the actual needs of clinicians. In complex surgical conditions, it may be difficult for surgeons to reappear the 3D visualization imaging in minds. Personalized 3D printed model can display the spatial relationship of each branch of the blood vessel and bronchus stereoscopically. By placing the 3D model in front of the display screen for real-time intraoperative reference, accidental injury of small vessels can be minimized, and the risk of surgery can be reduced (Figure 1F). It may explain that the blood loss in the 3D model group is significantly less than that in the other two groups.
A questionnaire survey was mainly aimed to evaluate the 3D printed model. The assessment was based on the subjective satisfaction survey of the operators. The role of 3D models in preoperative planning and intraoperative guidance is prominent (16). On the other hand, it is difficult to explain the characteristics of APL clearly by rough drawing because patients lack corresponding medical knowledge. It may play a negative role in patients’ choice of APL. The 3D model provides a useful communication medium for surgeons and patients. Also, through the intuitive perception and observation of the 3D model, young residents can quickly understand the pulmonary vascular anatomy, and shorten the learning curve. In the questionnaire survey, we also found that not all senior surgeons recognized the advantages of 3D models. Some experienced surgeons prefer to operate by referring to CT images and actual conditions during the operation. However, for complex pulmonary surgery, this is not the primary trend.
Expert perspective
Because of the complexity of thoracoscopic APL for early-stage NSCLC, novel cutting-edge technologies, including reconstruction software tools and 3D printing equipment, are needed to assist nodule localization, to recognize aberrant anatomy confirmation and decision-making on the extent of resection. While there is much evidence 3D reconstruction is superior to CT imaging alone, there is no current recommendation or consensus on selecting appropriated early-stage NSCLC patients for 3D reconstruction or 3D printing model. Here, we propose a preliminary score criterion based on the subjective questionnaire survey and live meeting discussion (Table 5). This pre-operative rating scale was created to optimize the clinical application of 3D technology for thoracoscopic APL. According to the total score of three items (segmentectomy position, CT imaging, and operator experience), we divided the grading of recommendations as following: (A) high recommendation for 3D reconstruction and moderate recommendation for 3D printing model (score: 4–6); (B) moderate recommendation for 3D reconstruction and optional for 3D printing model (score: 2–3); (C) low recommendation for 3D printing model and optional for 3D reconstruction (score: 0–1).

Full table
Personalized 3D printed model enhanced the confidence of surgeons to choose favorable decisions and perform complex surgery. The limitation of its popularization mainly comes from the increased expenses of patients and additional printing time (average 8–15 hours) (19,23). Considering the current restrictions, experts unanimously agreed that it is not suitable to prepare 3D printed models for all APLs. However, interrogating this technology through these different aspects will likely increase our present knowledge and improving surgeons’ ability to select the appropriate surgical mode in the future.
Conclusions
In summary, 3D reconstruction imaging and 3D printed model both have significant advantages in locating nodules and identifying vascular variations. For APL of complex segmentectomy, the 3D model has its unique advantages because of its excellent sense of space and distance. Meanwhile, it can be used in real-time intraoperative control to ensure the safety of APL, which is suitable for clinical application. A preliminary pre-operative rating scale was manuscripted to select appropriate patients for 3D reconstruction or 3D printing, and further researches are needed to develop detailed guideline for the application of this technique in thoracic surgery.
Acknowledgments
Funding: The study was funded by National Key R&D Program of China (2017YFC1308700), Institutional Fundamental Research Funds (2018PT32033) and ETHICON·Excellent in surgery grant (2018-011-ZZ).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-571
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-571
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-571
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-571). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (approval number: 191137-1921). The requirement of patients’ informed consent was waived owing to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schubert C, van Langeveld MC, Donoso LA. Innovations in 3D printing: a 3D overview from optics to organs. Br J Ophthalmol 2014;98:159-61. [Crossref] [PubMed]
- Liu X, Zhao Y, Xuan Y, et al. Three-dimensional printing in the preoperative planning of thoracoscopic pulmonary segmentectomy. Transl Lung Cancer Res 2019;8:929-37. [Crossref] [PubMed]
- Akiba T, Inagaki T, Nakada T. Three-dimensional printing model of anomalous bronchi before surgery. Ann Thorac Cardiovasc Surg 2014;20 Suppl:659-62. [Crossref] [PubMed]
- Fan Y, Wong RHL, Lee AP. Three-dimensional printing in structural heart disease and intervention. Ann Transl Med 2019;7:579. [Crossref] [PubMed]
- Noecker AM, Chen JF, Zhou Q, et al. Development of patient-specific three-dimensional pediatric cardiac models. Asaio J 2006;52:349-53. [Crossref] [PubMed]
- Sun X, Zhang H, Zhu K, et al. Curved section modeling-based three-dimensional printing for guiding septal myectomy. J Thorac Dis 2018;10:E535-7. [Crossref] [PubMed]
- Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016;159:1485-500. [Crossref] [PubMed]
- Ji Z, Sun H, Jiang Y, et al. Comparative study for CT-guided (125)I seed implantation assisted by 3D printing coplanar and non-coplanar template in peripheral lung cancer. J Contemp Brachytherapy 2019;11:169-73. [Crossref] [PubMed]
- Aokage K, Yoshida J, Hishida T, et al. Limited resection for early-stage non-small cell lung cancer as function-preserving radical surgery: a review. Jpn J Clin Oncol 2017;47:7-11. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33. [Crossref] [PubMed]
- Gao S, Qiu B, Li F, et al. Comparison of thoracoscopic anatomical partial-lobectomy and thoracoscopic lobectomy on the patients with pT1aN0M0 peripheral non-small cell lung cancer. Zhonghua Wai Ke Za Zhi 2015;53:727-30. [PubMed]
- Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604-11. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]
- Katayama H, Kurokawa Y, Nakamura K, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 2016;46:668-85. [Crossref] [PubMed]
- Valverde I, Gomez-Ciriza G, Hussain T, et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardiothorac Surg 2017;52:1139-48. [Crossref] [PubMed]
- Cantinotti M, Valverde I, Kutty S. Three-dimensional printed models in congenital heart disease. Int J Cardiovasc Imaging 2017;33:137-44. [Crossref] [PubMed]
- Zabaleta J, Aguinagalde B, Lopez I, et al. Creation of a multidisciplinary and multicenter study group for the use of 3D printing in general thoracic surgery: lessons learned in our first year experience. Med Devices (Auckl) 2019;12:143-9. [Crossref] [PubMed]
- Kurenov SN, Ionita C, Sammons D, et al. Three-dimensional printing to facilitate anatomic study, device development, simulation, and planning in thoracic surgery. J Thorac Cardiovasc Surg 2015;149:973-9.e1. [Crossref] [PubMed]
- Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgerydagger. Eur J Cardiothorac Surg 2014;46:e120-6. [Crossref] [PubMed]
- Kato H, Oizumi H, Suzuki J, et al. Thoracoscopic anatomical lung segmentectomy using 3D computed tomography simulation without tumour markings for non-palpable and non-visualized small lung nodules. Interact Cardiovasc Thorac Surg 2017;25:434-41. [Crossref] [PubMed]
- Xue L, Fan H, Shi W, et al. Preoperative 3-dimensional computed tomography lung simulation before video-assisted thoracoscopic anatomic segmentectomy for ground glass opacity in lung. J Thorac Dis 2018;10:6598-605. [Crossref] [PubMed]
- Smelt JLC, Suri T, Valencia O, et al. Operative Planning in Thoracic Surgery: A Pilot Study Comparing Imaging Techniques and Three-Dimensional Printing. Ann Thorac Surg 2019;107:401-6. [Crossref] [PubMed]