|
Cite this article as: Gao H, Ding X, Wei D, Cheng P, Su
X, Liu H, Aziz F, Wang D, Zhang T. Erlotinib in patients
with advanced non-small-cell lung cancer: A meta-analysis.
Transl Lung Cancer Res 2012;1(2):129-144. DOI: 10.3978/
j.issn.2218-6751.2012.06.01
Original Article
Erlotinib in patients with advanced non-small-cell lung cancer: A
meta-analysis
Hui Gao1†, Xin Ding2†, Dong Wei1, Peng Cheng1, Xiaomei Su1, Huanyi Liu1, Fahad Aziz3, Daoyuan
Wang1, Tao Zhang1
1Department of Oncology, 2Department of Neurology, PLA General Hospital of Chengdu Military Region, Chengdu 610083 PR China; 3Department
of Internal Medicine, Mount Sinai School of Medicine-Jersey City Campus, Jersey City, NJ, USA
†These authors contributed equally to this work
Corresponding to: Dr Tao Zhang, MD. Department of Oncology, PLA General Hospital of Chengdu Military Region, Tianhui Town, Jinniu District, Chengdu 610083, PR China. Email: drtao.zhang@gmail.com.
|
|
Abstract
Erlotinib is a potent reversible HER1/epidermal growth factor receptor (EGFR) tyrosine
kinase inhibitor with single-agent activity in patients with non–small-cell lung cancer (NSCLC). In this
article, we updated the evidence of erlotinib in treating advanced NSCLC by adding new results of RCTs
published between January 2011 and May 2012 into a pooled analysis which had been published in 2011.
Outcomes analyzed were objective response rate (ORR), progression free survival (PFS), overall survival
(OS) and adverse events. Twenty trials including 9,005 patients were identified, and six of them were
recently published. As first-line therapy compared to placebo or chemotherapy, there was a similar ORR
(P=0.29 and 0.42), PFS (P=0.09 and 0.25) and OS (P=0.73 and 0.49). However, for the patients with EGFR
mutations, erlotinib based regimens could significantly improve ORR (P<0.01), prolong PFS (P<0.0), but
did not prolong OS (P=0.22). As maintenance therapy compared with placebo, erlotinib based regimens
significantly increased ORR (P<0.01), prolonged PFS (P<0.01), but did not improve OS (P=0.22). As second/
third-line therapy comparing with placebo, erlotinib based regimens also significantly increased ORR
(P<0.01), prolonged PFS (P<0.01), and improved OS (P<0.01). As second/third-line therapy compared with
chemotherapy, gefitinib, or vandetanib, the outcomes were similar between two arms. However, compared
with PF299804, there was a decreased ORR (P=0.02), and shorten PFS (P=0.02). Meanwhile, The patients
treated with erlotinib based regimens suffered from more diarrhea, rash, and less fatigue, neutropenia, and
thrombocytopenia than other agent based regimens. Our meta analysis showed that erlotinib based regimens
could significantly increase ORR, improve PFS as first-line maintenance therapy or second/third-line
therapy comparing with placebo or PF299804.
Key words Erlotinib; advanced non-small-cell lung cancer; meta analysis
Submitted May 07, 2012. Accepted for publication Jun 06, 2012.
DOI: 10.3978/j.issn.2218-6751.2012.06.01 |
|
Introduction
Lung cancer is the major cause of cancer deaths worldwide,
and the majority of new cases belong to advanced non small
cell lung cancer (NSCLC) catagory ( 1). The standard firstline
treatment for advanced NSCLC is a platinum-based
two-drug combination regimen ( 2). However, no doublet
regimen has been proved superior, and survival outcomes
remained poor (median survival is 7.4 to 8.1 months; 1-year
survival rate is 28% to 47%) ( 3-5). Thus the development
of more effective therapy remains challenging. The
development of agents that target the epidermal growth
factor receptor signal transduction pathways has provided a
class of novel targeted therapeutic agents. The epidermal growth factor receptors (EGFR) have
shown to play a significant role in tumorigenesis, with up
to 80% of NSCLC expressing EGFR ( 6, 7). Overexpression of EGFR is associated with advanced disease and poor
survival ( 8). Erlotinib (Tarceva, OSI Pharmaceuticals) is
a highly potent reversible HER1/EGFR tyrosine kinase
inhibitor (EGFR-TKI) that has shown significant antitumor
activity in preclinical studies ( 9). The antitumor activity
with single-agent erlotinib has been proved by phase I/
II studies in previously treated patients ( 10). In a large
randomized, double-blind, placebo-controlled phase III
trial in previously treated patients with advanced NSCLC,
erlotinib significantly prolonged survival versus placebo [6.7
vs. 4.7 months; hazard ratio (HR), 0.70; P<0.001], delayed
disease progression, and delayed worsening of diseaserelated
symptoms ( 11). The most common adverse events
with single-agent erlotinib consisted of mild/moderate rash
and diarrhea. However, this is the only phase III trial which
have shown prolonged survival with an EGFR inhibitor in
advanced NSCLC. In other phase II and III trials, erlotinib
based regimens did not show superior to other agent based
regimens. In 2011, We had carried out a pooled analysis of randomized
controlled trials (RCTs) that compared erlotinib based
regimens with other agent based regimens between January
1997 and 2011 ( 12). In this article, we added the results
of RCTs which were recently published between January
2011 and May 2012 into the meta analysis, and updated the
evidence.
|
|
Materials and methods
The aim of this meta analysis was to review all published
and reported randomized controlled trials comparing
the erlotinib based regimens with other agent based
regimens. Both published and unpublished trials reported
between January 1997 and May 2012 were identified
through a computer-based search of the PubMed
database and abstracts from the past 13 conferences of
the American Society of Clinical Oncology and the past
13 conferences of the European Society for Medical
Oncology. The search strategy included the following
keywords variably combined: advanced or metastatic, non
small cell lung cancer or NSCLC, Erlotinib or Tarceva.
In addition, we searched trial registries and conference
proceedings. We also examined reference lists of original
articles, and contacted original trialists for possible
unpublished trials. The deadline for trial inclusion was
May 1, 2012.
Inclusion and exclusion criteria
The aim of this analysis was to evaluate objective response
rate (ORR), progression free survival (PFS), overall survival
(OS), and relevant grade 3/4 adverse events. If erlotinib
(E) alone or based combination therapy was included in a
randomized controlled trial (RCT), it was considered to be
eligible. Inclusion criteria for the trails included: (I) patients
were randomly assigned to treatment; (II) erlotinib or based
combination regimen was compared to other agent or based
combination regimen without confounding by other agents
or interventions; and (III) only patients with diagnosis
of advanced NSCLC were included. Trials with missing
adequate statistical analysis information were also excluded.
Assessment of the trials was carried out openly with the
instrument reported by Moher et al. ( 13), and there was no
significant difference observed among the trials. Therefore,
the result of the validity assessment was not considered in
this meta analysis. The following information was extracted from each report:
study design, regimen details, allocated patients, cause of
disease, race or ethnic group, ECOG performance status,
pathological subtype, prior chemotherapy, smoking status,
EGFR protein expression, median follow-up, HRs for the
whole study populations, and the year of reporting. Data
was independently extracted from each report by XM.
Su and HY. Liu, who were blinded to each other, using a
standardized data recording form. After extraction, data
was reviewed and compared by T. Zhang and P. Cheng
All data were checked for internal consistency, and any
disagreements were resolved by discussion among the
investigators. We also tried to contact principal investigators
of the trials to confirm or update both published and
unpublished data.
The primary endpoints in the meta analysis were OS and
PFS. The secondary endpoints were ORR and adverse
events. Except adverse events, all analyses were conducted
on an intention-to-treat (ITT) basis, and all randomly
assigned patients were included in the analyses according to the allocated treatment. We looked for heterogeneity
among the trials based on standard methods ( 14). The
DerSimonian and Laird Q statistic (Q test) was used to
test for the heterogeneity among trials ( 15). Begg’s funnel
plots ( 16) and Egger’s test ( 17) were used to detect possible
publication bias. Based on the results of the Q test, we
applied a random-effects model (primarily) to estimate the
summary HRs, ORs and their 95% confidence intervals
(CIs). If HRs or its 95% CIs could not be obtained from
reports, Crude logHR and its variance were calculated
according to the method proposed by Parma et al. ( 18).
To reduce reading errors, original survival curves were
digitalized and enlarged, and data extraction was based on
reading off electronic coordinates for each point of interest. All statistical analyses were conducted with Review
Manager V. 5.0.23 (Nordic Cochran Centre, Copenhagen,
Denmark). All statistical tests were two-sided, and P values
of 0.05 were considered to be statistically significant.
|
|
Results
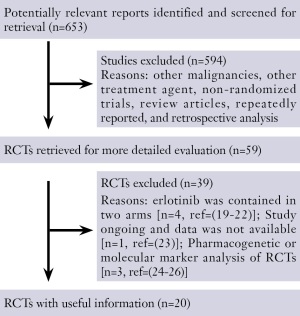
The flow chart of our study is shown in Figure 1 ( 19-26).
Ultimately, results of twenty randomized phase II or III
trials had been published or presented at major international
meetings were included in this analysis. Although we did not
limit language in the process of searching, all the trials were
published in English. All the twenty trials were randomized
controlled trials and the results were almost based on
intention to treat analysis except adverse events. There were
three PIs responded to our requests of confirming update
both published or unpublished data of the trials.
Characteristics of the twenty trials
The characteristics of the twenty trials are listed in Table 1.
Three phase III RCTs comparing with placebo as firstline
therapy ( 27-29), two phase III and four phase II RCT
comparing with chemotherapy as first-line therapy ( 30-35),
three phase III and one phase II RCTs comparing with
placebo as maintenance therapy ( 36-39); one phase III
RCT comparing with placebo as second/third-line therapy
( 11), one phase III and one phase II RCTs comparing with
chemotherapy as second/third-line therapy ( 40, 41), one
phase III RCT comparing with vandetanib as second/
third-line therapy ( 42), one phase II RCT comparing
with PF299804 as second/third-line therapy ( 43), and one
ohase II RCT comparing with Gefitinib as second/thirdline
therapy ( 44). In total, 9,005 patients were randomized
to receive erlotinib based regimens (4,620 patients) or
other agent based regimens (4,385 patients). 13 patients
enrolled in one trial were excluded after randomization ( 27).
Further information about unpublished data was obtained
by contacting the principal authors. No potential sources
of heterogeneity including sex, age, ECOG performance
status, pathological subtype, prior chemotherapy, smoking
status were associated with significant differences in
outcomes.
| Table 1 Characteristics of the twenty trials included in this meta analysis |
| Author |
Year |
Publication
form |
Design of
studys |
Pts |
Chemo/Target therapy
regimen |
Sex |
PS |
Age |
Stage |
Adenocarcinoma
(%) |
Smoking
History
(%) |
| Gatzemeier |
2007 |
Full text |
Phase III
Bouble-blind |
586
|
E 150 mg/d, per oral + G
1,250 mg/m2, d1,8 + DDP 80
mg/m2, d1, 6 cycles
|
78.0
|
99.8
|
60.0
|
99.6
|
38.0
|
-
|
| Bouble-blind |
586
|
Placebo + G 1,250 mg/m2,
d1,8 + DDP 80 mg/m2, d1, 6
cycles |
75.0 |
99.8 |
59.1 |
99.8 |
38.0 |
- |
| Herbst |
2005 |
Full text |
Phase III |
539 |
E 150 mg/d, per oral + C
AUC 6, d1 + T 200 mg/m2,
d1, 6 cycles |
61.6 |
100 |
62.7 |
100 |
59.9 |
86.6 |
| - |
540 |
Placebo + C AUC 6, d1 + T
200 mg/m2, d1, 6 cycles |
59.7 |
99.8 |
62.6
|
100 |
61.4 |
91.8 |
| Lee |
2010 |
Abstract |
Phase III |
350 |
E 150 mg/d, per oral |
61.0 |
16 |
77.4 |
100 |
38 |
95.0 |
| - |
320 |
Placebo |
61.0 |
16 |
77.2 |
100 |
38 |
94.0 |
| Rosell |
2012 |
Full text |
Phase III |
86 |
E 150 mg/d, per oral |
67.0 |
86.0 |
65.0 |
98.0 |
95.0 |
34.0 |
| Open-label |
87 |
G 1,250 mg/m2,/, d1,8 (D 75
mg/m2, d1) + DDP 75 mg/m2
(C AUC 5), d1, 3 cycles |
78.0 |
86.0 |
65.0 |
100 |
90.0 |
28.0 |
| Zhou |
2011 |
Full text |
Phase III |
83 |
E 150 mg/d, per oral |
41.0 |
91.0 |
57.0 |
100 |
88.0 |
28.0 |
| Open-label |
82 |
G 1,000 mg/m2, d1,8 + C
AUC 5, d1, 4 cycles |
40.0 |
96.0 |
59.0 |
100 |
86.0 |
31.0 |
| Gridelli |
2011 |
Full text |
Phase II |
29 |
E 150 mg/d, per oral + S
400mg/d, per oral, bid |
59.0 |
100 |
76.0 |
100 |
86.0 |
93.0 |
| Open-label |
31 |
G 1,250 mg/m2, d1,8, 6
cycles + S 400 mg/d, per
oral, bid |
65.0 |
94.0 |
74.0 |
100 |
81.0 |
90.0 |
| Lilenbaum |
2008 |
Full text |
Phase II |
52 |
E 150 mg/d, per oral |
44.0 |
0 |
51.0 |
100 |
50.0 |
88.0 |
| Open-label |
51 |
C AUC 6, d1 + T 200 mg/m2,
d1, 6 cycles |
55.0 |
0 |
52.0 |
100 |
63.0 |
92.0 |
| Reck |
2010 |
Abstract |
Phase II |
144 |
E 150 mg/d, per oral |
65.0 |
100 |
75.5 |
100 |
50.0 |
82.0 |
| Open-label |
140 |
C AUC 5, d1 + NVB 25 mg/
m2, d1,8, 6 cycles |
| 100 |
76.1 |
99.0 |
49.0 |
86.0 |
| Chen |
2012 |
Full text |
Phase II |
57 |
E 150 mg/d, per oral |
82.5 |
80.7 |
78.1 |
100 |
63.2 |
79.0 |
| Open-label |
56 |
NVB 60 mg/m2, d1,8, 6
cycles |
80.4 |
73.2 |
77.8 |
100 |
66.1 |
78.6 |
| Cappuzzo |
2010 |
Full text |
Phase III |
438 |
After CT, E 150 mg/d, per |
73.0 |
31.0 |
60.0 |
100 |
47.0 |
82.0 |
| Double-blind |
451 |
oral, After CT, Placebo |
75.0 |
32.0 |
60.0 |
100 |
44.0 |
83.0 |
| Miller |
2009 |
Abstract |
Phase III |
370 |
After CT, E 150 mg/d, per
oral + B 15 mg/kg, d1, q3
wks |
52.0 |
100 |
64.0 |
100 |
81.3 |
83.5 |
| Bouble-blind |
373 |
After CT, Placebo +
B 15 mg/kg, d1, q3 wks |
52.3 |
99.7 |
64.0 |
100 |
82.5 |
82.3 |
| Mok |
2010 |
Full text |
Phase II |
76 |
E 150 mg/d, per oral, d15-
28 + G 1,250 mg /m2, d1, 8
+ DDP 75 mg/m2 (C AUC 5),
d1, 6 cycles |
71.0 |
100 |
57.0 |
100 |
67.0 |
68.0 |
| Bouble-blind |
78 |
Placebo+G 1,250 mg/m2,
d1,8 + DDP 75 mg/m2 (C
AUC 5), d1, 6 cycles |
69.0 |
100 |
57.5 |
100 |
67.0 |
64.0 |
| Perol |
2010 |
Abstract |
Phase III |
155 |
After CT, E 150 mg/d, per
oral |
73 |
100 |
56.4 |
100 |
63 |
- |
| Open-label |
155 |
After CT, Observation |
73 |
100 |
59.8 |
100 |
67 |
- |
| Shepherd |
2005 |
Full text |
Phase III |
488 |
E 150 mg/d, per oral |
64.5 |
91.4 |
62.0 |
100 |
50.4 |
73.4 |
| Bouble-blind |
243 |
Placebo |
65.8 |
91.4 |
59.0 |
100 |
49.0 |
77.0 |
| Ciuleanu |
2012 |
Full text |
Phase III |
203 |
E 150 mg/d, per oral |
79.0 |
81.0 |
59.0 |
100 |
47.0 |
85.0 |
| Open-label |
221 |
D or M |
72.0 |
79.0 |
59.0 |
100 |
52.0 |
80.0 |
| Herbst |
2007 |
Full text |
Phase II |
39 |
E 150 mg/d, per oral+B 15
mg/kg, d1, q3 wks |
43.6 |
100 |
68.0 |
100 |
82.1 |
84.6 |
| Open-label |
40 |
T 75 mg/m2, d1/M 500 mg/
m2, d1+B 15 mg/kg, d1, q3
wks |
57.5 |
100 |
63.5 |
100 |
75.0 |
90.0 |
| Vamvakas |
2010 |
Abstract |
Phase III |
166 |
E 150 mg/d, per oral |
81.3 |
79.2/td>
| 65 |
100 |
53.6 |
- |
| Open-label |
166 |
MTA 500 mg/m2, d1, q3 wks |
82.5 |
81.3 |
66 |
100 |
56.6 |
- |
| Natale |
2011 |
Full text |
Phase III |
617 |
E 150 mg/d, per oral |
64.0 |
88.0 |
61.0 |
100 |
57.0 |
76.0 |
| Bouble-blind |
623 |
V 300 mg/d, per oral |
61.0 |
99.0 |
60.0 |
100 |
63.0 |
79.0 |
| 2010 |
Abstract |
Phase II |
94 |
E 150 mg/d, per oral |
59.6 |
96.8 |
67.0 |
100 |
64.9 |
78.7 |
| | Open-label |
94 |
PF299804 45 mg/do, per
oral |
58.5 |
81.9 |
69.0 |
100 |
66.0 |
79.8 |
| Kim |
2011 |
Full text |
Phase II |
48 |
E 150 mg/d, per oral |
14.6 |
85.4 |
56.0 |
83.4 |
89.6 |
4.2 |
| Open-label |
48 |
Gefitinib 250 mg/d, per oral |
14.6 |
85.4 |
60.0 |
87.5 |
91.7 |
8.3 |
| All trials were phase III trials except for Gridelli’s, Lilenbaum’s, Reck’s, Mok’s, and Herbst’s trials which were designed as phase II trials.
A, abstract; AUC, area under the serum concentration-time curve; B, bevacizumab; C, carboplatin; CT, chemotherapy; D, docetaxel;
DDP, cisplatin; E, erlotinib; F, full text; G, gemcitabine; M, pemetrexed; NVB, vinorelbine; Pts, patients; PS, performance status; S,
Sorafenib; T, paclitaxel; V, vandetanib ( a targeted drug); d, day; po, per oral; wks, weeks |
Seventeen trials except for Lee’s, Miller’s, and Perol’s trials
reported ORR ( 29, 37, 39). The response rates ranged
from 4.0% to 82.9% for the erlotinib based regimens and
from <1.0% to 47.9% for the other agent based regimens
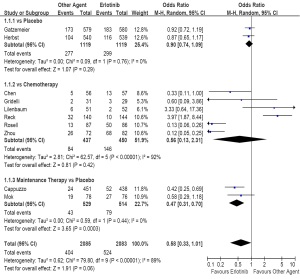
( Table 2). As first-line therapy, including ten trials and
4,168 patients (erlotinib, n=2,083; other agent, n=2,058),
the random-effects model pooled estimate evaluated for
ORR showed a similar ORR for erlotinib based regimens
(OR, 0.58; 95% CI, 0.33 to 1.01; P=0.06). However, the
test for heterogeneity showed a significant difference
(I2=89%, P<0.01), so we had to carry out subgroup analysis.
The subgroup analysis showed a similar ORR comparing with placebo (OR, 0.90; 95% CI, 0.74 to 1.09; P=0.29),
or chemotherapy (OR, 0.56; 95% CI, 0.13 to 2.31;
P=0.42), but an increased ORR comparing with placebo
as maintenance therapy (OR, 0.47; 95% CI, 0.31 to 0.70;
P<0.01; Figure 2). Two of the six trials comparing with chemotherapy as
first line therapy only enrolled the patients with EGFR
mutations ( 32, 33). So, there was a significant heterogeneity
in this subgroup (I2=92%, P<0.01). For these patients,
erlotinib based regimens could significantly improve the
ORR than chemotherapy (OR, 0.12; 95% CI, 0.07 to 0.20;
P<0.01; data not shown).
| Table 2 Responses in the seventeen trials |
| Author |
Chemo/Targeted therapy
regimen |
Pts with complete
or partial response |
Randomized Pts |
Objective response rate
(%) |
| Gatzemeier et al. |
E+G+DDP |
183 |
580 |
31.5 |
| P+G+DDP |
173 |
579 |
29.9 |
| Herbst et al. |
E+C+T |
116 |
539 |
21.5 |
| P+C+T |
104 |
540 |
19.3 |
| Rosell zffigure. |
E |
50 |
86 |
58.1 |
| G (D) + DDP (C) |
13 |
87 |
14.9 |
| Zhou et al. |
E |
68 |
82 |
82.9 |
| G + C |
26 |
72 |
36.1 |
| Gridelli et al.. |
E + S |
3 |
29 |
10.3 |
| G + S |
2 |
31 |
6.5 |
| Lilenbaum et al.. |
E |
2 |
52 |
4.0 |
| C+T |
6 |
51 |
12.0 |
| Reck et al.. |
E |
10 |
144 |
6.9 |
| C+NVB |
32 |
140 |
22.9 |
| Chen et al.. |
E |
13 |
57 |
22.8 |
| C+NVB |
5 |
56 |
8.9 |
| Cappuzzo et al.. |
After CT, E |
52 |
438 |
11.9 |
| After CT, P |
24 |
451 |
5.3 |
| Mok et al.. |
E+G+DDP (C) |
27 |
76 |
35.5 |
| P+G+DDP (C) |
19 |
78 |
24.4 |
| Shepherd et al.. |
E |
38 |
488 |
7.8 |
| P |
2 |
243 |
<1 |
| Ciuleanu et al.. |
E |
16 |
203 |
7.9 |
| D or M |
14 |
221 |
6.3 |
| Herbst et al.. |
E+B |
12 |
39 |
30.8 |
| T/M+B |
16 |
40 |
40.0 |
| Vamvakas et al.. |
E
13 |
166 |
8 |
| MTA |
19 |
166 |
11.4 |
| Natale et al. |
E |
74 |
617 |
12.0 |
| V |
75 |
623 |
12.0 |
| Boyer et al.. |
E |
4 |
94 |
4.3 |
| PF299804 |
16 |
94 |
17.0 |
| Kim et al.. |
E |
19 |
48 |
39.6 |
| Gefitinib |
23 |
48 |
47.9 |
| B, bevacizumab; C, carboplatin; D, docetaxel; DDP, cisplatin; E, erlotinib; G, gemcitabine; M, pemetrexed; NVB, vinorelbine;
P, Placebo; Pts, patients; S, Sorafenib; T, paclitaxel; V, vandetanib ( a targeted drug). Response Rate was not included in the
objectives of Lee’s, Miller’s, and Perol’s studys |
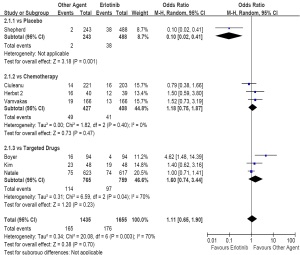
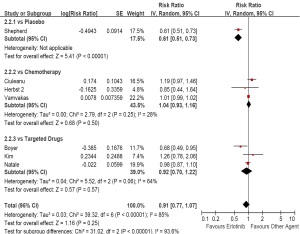
As second/third-line therapy including seven trials and
3,090 patients (erlotinib, n=1,655; other agent, n=1,435),
the pooled estimate showed a similar ORR for erlotinib
based regimens (OR, 1.11; 95% CI, 0.65 to 1.90; P=0.70).
The test for heterogeneity also showed a significant
difference (I2=70%, P<0.01). When compared with placebo,
the subgroup analysis showed an increased ORR (OR, 0.10;
95% CI, 0.02 to 0.41; P<0.01). However, compared with
chemotherapy, there was a similar ORR between two arms
(OR, 1.18; 95% CI, 0.75 to 1.87; P=0.47; Figure 3). With respect to all efficacy outcomes, random-effect
( Figure 2, 3, 4, 5, 6, 7) and fixed-effects models (data not
shown) yielded virtually identical results. Neither a Begg’s
funnel plot nor a rank correlation test regarding response
rate indicated the existence of publication bias (Z=0.21,
P=0.84). The results of Egger’ test was similar.
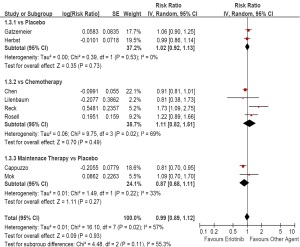
Progression free survival
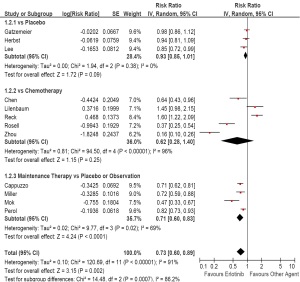
Ninteen trials except for Gridelli’s trial reported PFS
( Table 3) ( 34). As first-line therapy, the random-effects
model pooled estimate evaluated for PFS showed a
improved PFS for erlotinib based regimens (HR, 0.73;
95% CI, 0.60 to 0.89; P<0.01). However, the test for
heterogeneity showed a significant difference (I 2=91%,
P<0.01), so we had to carry out subgroup analysis. The
pooled estimate showed a similar PFS comparing with
placebo (HR, 0.93; 95% CI, 0.85 to 1.01; P=0.09),
and chemotherapy (HR, 0.62; 95% CI, 0.28 to 1.40;
P=0.25), but a prolonged PFS comparing with placebo
as maintenance therapy (HR, 0.71; 95% CI, 0.60 to 0.83;
P<0.01; Figure 4).
| Table 3 Progression free survival and overall survival in the twenty trials |
| Author |
Chemo/Target therapy
regimen |
ITT analysis |
Randomized Pts |
Median PFS
(month) |
P Value |
Median OS
(month) |
P Value |
| Gatzemeier et al. |
E+G+DDP |
Yes |
586 |
5.50 |
0.74 |
10.00 |
0.49 |
| P+G+DDP |
586 |
5.80 |
10.90 |
| Herbst et al. |
E+C+T |
Yes |
539 |
5.10 |
0.36 |
10.60 |
0,95 |
| P+C+T |
540 |
4.90 |
10.50 |
| Lee et al. |
E |
Yes |
350 |
2.8 |
0.038 |
3.8 |
0.069 |
| P |
320 |
2.7 |
3.6 |
| Rosell et al. |
E |
Yes |
86 |
9.7 |
<0.0001 |
19.3 |
0.87 |
| G (D) + DDP (C) |
87 |
5.2 |
19.5 |
| Zhou et al. |
E |
No |
83 |
13.1 |
<0.0001 |
- |
- |
| G+C |
82 |
4.6 |
|
| Gridelli et al. |
E+S |
Yes |
29 |
3.0 |
- |
12.6 |
- |
| G+S |
31 |
2.0 |
6.6 |
| Lilenbaum et al. |
E |
Yes |
52 |
1.90 |
0.063 |
6.60 |
0.018 |
| C+T |
51 |
3.50 |
9.70 |
| Reck et al. |
E |
No |
125 |
2.4 |
0.001 |
7.9 |
0.21 |
| C+NVB |
113 |
4.6 |
8.4 |
| Chen et al. |
E |
Yes |
57 |
4.57 |
0.029 |
11.67 |
0.698 |
| NVB |
56 |
2.53 |
9.3 |
| Cappuzzo et al. |
After CT, E |
Yes |
437 |
2.87 |
<0.01 |
12.0 |
0.009 |
| After CT, P |
447 |
2.59 |
11.0 |
| Miller et al. |
After CT, E+B |
Yes |
373 |
4.76 |
0.001 |
|
- |
| After CT, P+B |
370 |
3.75 |
|
| Mok et al. |
E+G+DDP (C) |
Yes |
76 |
6.86 |
<0.01 |
17.29 |
0.72 |
| P+G+DDP (C) |
78 |
5.46 |
17.67 |
| Perol et al. |
After CT, E |
No |
153 |
2.9 |
0.002 |
|
- |
| After CT, Observation |
152 |
1.9 |
|
| Shepherd et al. |
E |
Yes |
488 |
2.20 |
<0.01 |
6.70 |
<0.01 |
| P |
243 |
1.80 |
4.70 |
| Ciuleanu et al. |
E |
Yes |
203 |
1.47 |
0.089 |
5.3 |
0.55 |
| D or M |
221 |
2.01 |
5.3 |
| Herbst et al. |
E+B |
Yes |
39 |
4.40 |
>0.05 |
13.70 |
>0.05 |
| T/M+B |
40 |
4.80 |
12.60 |
| Vamvakas et al. |
E |
Yes |
166 |
3.6 |
0.30 |
7.9 |
0.92 |
| MTA |
166 |
2.7 |
8.9 |
| Natale et al. |
E |
Yes |
617 |
2.08 |
0.72 |
7.8 |
0.83 |
| V |
623 |
2.64 |
6.9 |
| Boyer et al. |
E |
Yes |
94 |
1.94 |
0.019 |
- |
- |
| PF299804 |
94 |
2.89 |
|
| Kim et al. |
E |
Yes |
48 |
3.1 |
0.336 |
- |
- |
| Gefitinib |
48 |
4.9 |
|
| B, bevacizumab; C, carboplatin; D, docetaxel; DDP, cisplatin; E, erlotinib; G, gemcitabine; ITT, intention to treat; M, pemetrexed;
MTA, Pemetrexed; P, Placebo; PFS, progression free survival; Pts, patients; S, Sorafenib; T, paclitaxel; V, vandetanib |
For the patients with EGFR mutations ( 32, 33), our
analysis showed that erlotinib based regimens could
significantly improve the PFS than chemotherapy (HR, 0.25;
95% CI, 0.11 to 0.56; P<0.01; data not shown). As second/third-line therapy including seven trials, the
pooled estimate showed a similar PFS for erlotinib based
regimens (HR, 0.91; 95% CI, 0.77 to 1.07; P=0.25). The
test for heterogeneity also showed a significant difference
(I2=85%, P<0.01). The subgroup analysis showed a
prolonged PFS compared with placebo (HR, 0.61; 95%
CI, 0.51 to 0.73; P<0.01), but a similar PFS compared with
chemotherapy (HR, 1.04; 95% CI, 0.93 to 1.16; P=0.50;
Figure 5). Neither a Begg’s funnel plot nor a rank correlation
test regarding response rate indicated the existence of
publication bias (Z=0.70, P=0.48). The results of Egger’ test
was similar.
Only thirteen trials reported OS ( Table 3). As first-line
therapy including eight trials, the random-effects model
pooled estimate evaluated for OS showed a similar OS for
erlotinib based regimens (HR, 0.99; 95% CI, 0.89 to 1.22;
P=0.93). The test for heterogeneity showed a significant
difference (I2=57%, P=0.02). The subgroup analysis showed
a similar OS compared with placebo (HR, 1.02; 95% CI,
0.92 to 1.13; P=0.73), or with chemotherapy (HR, 1.11;
95% CI, 0.82 to 1.51; P=0.49), or as maintenance therapy
(HR, 0.87; 95% CI, 0.68 to 1.11; P=0.22; Figure 6). Only one trial reported OS for the patients with EGFR
mutations ( 32). Our analysis showed that there was a similar
OS between erlotinib based regimens and chemotherapy
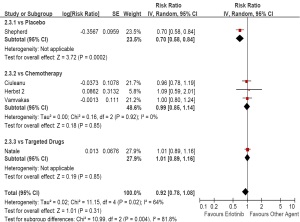
(HR, 1.22; 95% CI, 0.89 to 1.66; P=0.22; data not shown). As second/third-line therapy including five trials, the
pooled estimate showed a similar OS for erlotinib based
regimens (HR, 0.92; 95% CI, 0.78 to 1.08; P=0.31). The
test for heterogeneity showed a significant difference
(I 2=64%, P=0.02). The subgroup analysis showed a
prolonged OS compared with placebo (HR, 0.70; 95%
CI, 0.58 to 0.84; P<0.01), but a similar OS compared with
chemotherapy (HR, 0.99; 95% CI, 0.85 to 1.14; P=0.85).
Erlotinib could also not improve OS of the patients
compared with vandetanib (HR, 1.01; 95% CI, 0.89 to 1.16;
P=0.85; Figure 7). Neither a Begg’s funnel plot nor a rank correlation test regarding response rate indicated the existence of
publication bias (Z=0.43, P=0.70). The results of Egger’ test
was similar.
Ninteen trials including 8,147 patients, except Chen’
trial, provided results of adverse events ( 35). Reported
toxicities were analyzed in only sixteen trials except for the
targeted drugs containing trials ( 37, 38) ( Table 4). Grade
3/4 diarrhea (OR, 5.08; 95% CI, 3.43 to 7.52; P<0.01) and
rash (OR, 19.37; 95% CI, 11.40 to 32.92; P<0.01) were
significantly prominent in the erlotinib based regimens,
with all intertrial variability consistent with the play of
chance. However, fatigue (OR, 0.72; 95% CI, 0.55 to 0.94;
P=0.02), neutropenia (OR, 0.74; 95% CI, 0.59 to 0.92;
P<0.01) and thrombocytopenia (OR, 0.73; 95% CI, 0.57 to
0.93; P=0.01) were significantly decreased in the erlotinib
based regimens. Compared to other agent based regimens,
erlotinib based regimen did not increase the frequency
of other adverse events. The heterogeneity test found no
statistical significance except for thrombocytopenia.
| Table 4 Adverse events in trials comparing erlotinib based regimen with other agent based regimen (grades III and IV) |
| Adverse events |
No. of
evaluable
trials |
Erlotinib based therapy |
Other agent based therapy |
OR (95% CI) |
P Value for Q Test |
| Pts with adverse events |
Evaluable
Pts |
Pts with adverse
events |
Evaluable
Pts |
| Diarrheaa |
16 |
149 |
3445 |
27 |
3182 |
5.08 (3.43-7.52) |
<0.01 |
| Rasha |
16 |
261 |
3445 |
8 |
3182 |
19.37 (11.40-32.92) |
<0.01 |
| Fatiguea |
11 |
105 |
2181 |
124 |
1916 |
0.72(0.55-0.94) |
0.02 |
| Neutropeniaa |
11 |
174 |
2042 |
225 |
2052 |
0.74(0.59-0.92) |
<0.01 |
| Thrombocytopeniaa |
10 |
116 |
1774 |
153 |
1756 |
0.73(0.57-0.93) |
0.01 |
| Anemia |
12 |
137 |
1810 |
115 |
1807 |
1.21(0.93-1.57) |
0.15 |
| Nausea/vomiting |
11 |
112 |
2263 |
113 |
2000 |
0.96(0.73-1.26) |
0.76 |
| Anorexia |
10 |
44 |
2044 |
31 |
1791 |
1.24 (0.78-1.97) |
0.36 |
| Arthralgia/myalgia |
4 |
7 |
541 |
11 |
554 |
0.64(0.25-1.62) |
0.35 |
| Heterogeneity tests showed no significant results except for thrombocytopenia. OR, odds ratio; CI, confidence interval; athe result
had a significant difference |
Because of the significant heterogeneity (data not
showen), we had to compare erlotinib with other targeted
drugs respectively ( Figure 5, 6, 7). Compared with gefitinib,
there was a similar ORR (OR, 1.40; 95% CI, 0.62 to 3.61;
P=0.41), PFS (HR, 1.26; 95% CI, 0.78 to 2.06; P=0.35),
and the frequency of grade 3/4 adverse events (data not
showen). Compared with vandetanib, there was a similar
ORR (OR, 1.00; 95% CI, 0.71 to 1.40; P=0.98), PFS (HR,
0.98; 95.22% CI, 0.87 to 1.10; P=0.72), OS (HR, 1.01;
95.08% CI, 0.89 to 1.16; P=0.83), and the frequency of
grade 3/4 adverse events (data not showen). Compared with
PF299804, there was a decreased ORR (OR, 3.87; 95% CI,
1.27 to 11.81; P=0.02), and shorten PFS (HR, 0.58; 95% CI,
0.49 to 0.95; P=0.02). At the same time, erlotinib did not
increase the frequency of grade 3/4 adverse events except
for diarrhea (OR, 0.25; 95% CI, 0.07 to 0.91; P=0.04).
|
|
Discussion
The epidermal growth factor receptor (EGFR) family is
part of a complicated signal-transduction network that is a
key to several critical cellular processes ( 39). Overexpression
of EGFR is common in non small cell lung cancer (NSCLC)
and is associated with poor survival. During the last decade,
the treatment for patients with advanced NSCLC has
improved as a result of the invention of novel, effective,
targeting the EGFR pathway agents such as gefitinib and
erlotinib. Up to now, the reports of several phase II/III
trials showed inconsistent results on clinical outcomes with
regard to ORR, PFS, and OS. Thus, the impact of erlotinib
based regimens on the survival of advanced NSCLC
patients compared with other agent based regimens
remained undetermined. In this meta analysis, we identified twenty RCT trials
including 9,005 patients, and the largest accounted for
1,240 randomly assigned patients. However, because of
the difference of the schedule of treatment and controlled
regimens, the heterogeneity between trials was statistically
significant. Thus we must explain the results with caution
and we had to carry out subgroup analysis according to the
schedule of treatment and controlled regimens. As first-line
therapy compared to placebo or chemotherapy, there were
similar PFS (P=0.09 and 0.25) and OS (P=0.73 and 0.49).
However, for the patients with EGFR mutations, erlotinib
based regimens could significantly improve ORR (OR, 0.12;
95% CI, 0.07 to 0.20; P<0.01), prolong PFS (HR, 0.25; 95%
CI, 0.11 to 0.56; P<0.0), but not OS (HR, 1.22; 95% CI, 0.89
to 1.66; P=0.22). As maintenance therapy compared with
placebo, erlotinib based regimens significantly increased
ORR (OR, 0.47; 95% CI, 0.31 to 0.70; P<0.01), prolonged
PFS (HR, 0.71; 95% CI, 0.60 to 0.83; P<0.01), but did not improve OS (HR, 0.87; 95% CI, 0.68 to 1.11; P=0.22). As
second/third-line therapy comparing with placebo, erlotinib
based regimens also significantly increased ORR (OR, 0.10;
95% CI, 0.02 to 0.41; P<0.01), prolonged PFS (HR, 0.61;
95% CI, 0.51 to 0.73; P<0.01), and improved OS (HR, 0.70;
95% CI, 0.58 to 0.84; P<0.01). However, as second/thirdline
therapy compared with chemotherapy, the outcomes
were similar between two arms.
As first-line therapy, from the results of this meta
analysis, we found that no matter compared with placebo
or chemotherapy, for the patients we did not know their
status of EGFR mutations, erlotinib based regimens could
not increase ORR, improve PFS and OS. However, For the
patients with EGFR mutations, erlotinib based regimens
could significantly improve ORR, prolong PFS, but not OS.
As first line maintenance therapy, we should prefer erlotinib
to placebo.
As second/third-line therapy, we should prefer erlotinib
or chemotherapy to best supportive care (BSC) in some
patients with good PS status. Compared with molecular
targeted drugs such as gefitinib or vandetanib, there was
no significant difference between two arms. However,
compared with PF299804, there was a decreased ORR (OR,
3.87; 95% CI, 1.27 to 11.81; P=0.02), and shorten PFS (HR,
0.58; 95% CI, 0.49 to 0.95; P=0.02).
In the pooled analysis published in 2011, an unexpected
finding was an increased incidence in anemia with the
erlotinib combination ( 12). At that time, we found that
this increase was mostly due to the result reported by
Gatzemeier’s trial ( 27), and believed this increased incidence
was just an accident and pointless. In this analysis, there
was not significant difference in the incidence of anemia
between erlotinib based regimens and other agent based
regimens. Neither the Begg’s funnel plot for publication
bias nor did the heterogeneity test yield a significant result.
Because the results based on fixed effect model were similar
to the results based on random effect model, we did not
show the results based on fixed effect model. However, there were still several limitations in this meta
analysis. First, this analysis was based on literature abstract based (AD) data, not individual patient data (IPD). An
IPD meta-analysis would give a more robust estimate
of the association but have to take a long time to obtain
data ( 45). But the analysis based on published trials is an
accepted method, offers the most comprehensive insight
into erlotinib based regimens as soon as possible and may
help physicians and their patients worldwide to make a
better informed decision regarding the most appropriate
therapy. A recently reported analysis confirmed that
individual patient-based (IPD) and literature abstractbased
(AD) meta-analyses did not differ substantially in
their outcome ( 46). Second, although we included twenty
trials, there were only one to six trials in each subgroup.
However, all the twenty trials were randomized
controlled trials, and all the results except for adverse
events were based on intention to treat analysis.
Therefore we considered our meta analysis based on
these trials is believable. Third, possible publication bias
is also a potential threat in our study, though we did not
detect it statistically. In conclusion, we updated the evidence of randomized
trials of erlotinib based regimens versus other agent based
regimens in treating advanced NSCLC. Although there are
some limitations, our findings demonstrate that erlotinib
based regimens significantly increase ORR, improve PFS as
first-line maintenance therapy or second/third line therapy
comparing with placebo. Thus, the use of erlotinib may be
a new effective therapy of treating advanced NSCLC as
first-line maintenance therapy, or second/third line therapy
compared with best supportive care (BSC).
|
|
Acknowledgements
Disclosure: The authors declare no conflict of interest.
|
|
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008.
CA Cancer J Clin 2008;58:71-96.
- Hotta K, Matsuo K, Ueoka H, et al. Addition of platinum
compounds to a new agent in patients with advanced nonsmall-
cell lung cancer: a literature based meta-analysis of
randomised trials. Ann Oncol 2004;15:1782-9.
- Schiller Jh, Harrington D, Belani Cp, et al. Comparison of
four chemotherapy regimens for advanced non-small-cell
lung cancer. N Engl J Med 2002;346:92-8.
- Depierre A, Chastang C, Quoix E, et al. Vinorelbine
versus vinorelbine plus cisplatin in advanced non-small cell
lung cancer: a randomized trial. Ann Oncol 1994;5:37-42.
- Negoro S, Masuda N, Takada Y, et al. Randomised phase
III trial of irinotecan combined with cisplatin for advanced
non-small-cell lung cancer. Br J Cancer 2003;88:335-41.
- Fontanini G, De Laurentiis M, Vignati S, et al. Evaluation
of epidermal growth factor-related growth factors and
receptors and of neoangiogenesis in completely resected
stage I-IIIA non-small-cell lung cancer: amphiregulin and
microvessel count are independent prognostic indicators
of survival. Clin Cancer Res 1998;4:241-9.
- Grünwald V, Hidalgo M. Developing inhibitors of the
epidermal growth factor receptor for cancer treatment. J
Natl Cancer Inst 2003;95:851-67.
- Douillard Jy, Shepherd Fa, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in
previously treated non-small-cell lung cancer: data from
the randomized phase III INTEREST trial. J Clin Oncol
2010;28:744-52.
- Dai Q, Ling Yh, Lia M, et al. Enhanced sensitivity to the
HER1/epidermal growth factor receptor tyrosine kinase
inhibitor erlotinib hydrochloride in chemotherapy-resistant
tumor cell lines. Clin Cancer Res 2005;11:1572-8.
- Perez-Soler R. Phase II clinical trial data with the
epidermal growth factor receptor tyrosine kinase inhibitor
erlotinib (OSI-774) in non-small-cell lung cancer. Clin
Lung Cancer 2004;6:S20-3.
- Shepherd Fa, Rodrigues Pereira J, Ciuleanu T, et al.
Erlotinib in previously treated non-small-cell lung cancer.
N Engl J Med 2005;353:123-32.
- Gao H, Ding X, Wei D, et al. Efficacy of erlotinib in
patients with advanced non-small cell lung cancer: a
pooled analysis of randomized trials. Anticancer Drugs
2011;22:842-52.
- Moher D. Jadad Ar, Nichol G, et al. Assessing the quality
of randomized controlled trials: an annotated bibliography
of scales and checklists. Control Clin Trials 1995;16:62-73.
- Higgins JP, Thompson SG. Quantifying heterogeneity in
a meta-analysis. Stat Med 2002;21:1539-58.
- Dersimonian R, Laird N. Meta-analysis in clinical trials.
Control Clin Trials. 1986;7:177-88.
- Begg CB, Mazumdar M. Operating characteristics of
a rank correlation test for publication bias. Biometrics
1994;50:1088-101.
- Egger M, Davey Smith G, Schneider M, et al. Bias in
meta-analysis detected by a simple, graphical test. BMJ
1997;315:629-34.
- Mk P, Torri V, Stewart L. Extracting summary statistics
to perform meta-analyses of the published literature for
survival endpoints. Stat Med 1998;17:2815-34.
- Riely GJ, Rizvi NA, Kris MG, et al. Randomized phase
II study of pulse erlotinib before or after carboplatin and
paclitaxel in current or former smokers with advanced
non-small-cell lung cancer. J Clin Oncol 2009;27:264-70.
- Gridelli C, Butts C, Ciardiello F, et al. An international,
multicenter, randomized phase III study of first-line
erlotinib followed by second-line cisplatin/gemcitabine
versus first-line cisplatin/gemcitabine followed by secondline
erlotinib in advanced non-small-cell lung cancer:
treatment rationale and protocol dynamics of the TORCH
trial. Clin Lung Cancer 2008;9:235-8.
- Ramalingam SS, Spigel DR, Chen D, et al. Randomized
phase II study of erlotinib in combination with placebo
or R1507, a monoclonal antibody to insulin-like growth
factor-1 receptor, for advanced-stage non-small-cell lung
cancer. J Clin Oncol 2011;29:4574-80.
- LeCaer H, Barlesi F, Corre R, et al. A multicentre phase II
randomised trial of weekly docetaxel/gemcitabine followed
by erlotinib on progression, vs the reverse sequence, in
elderly patients with advanced non small-cell lung cancer
selected with a comprehensive geriatric assessment (the
GFPC 0504 study). Br J Cancer 2011;105:1123-30.
- Stinchcombe T, Bradford DS, Lee CB, et al. Preliminary
results of a randomized phase II trial of first-line treatment
of gemcitabine (G) versus erlotinib (E) versus gemcitabine
and erlotinib (GE) in patients 70 years or older with
advanced non-small cell lung cancer (NSCLC). J Clin
Oncol 28:15s,2010 (suppl; abstr 7576).
- Liu G, Cheng D, Ding K, et al. Pharmacogenetic analysis
of BR.21, a placebo-controlled randomized phase III
clinical trial of erlotinib in advanced non-small cell lung
cancer. J Thorac Oncol 2012;7:316-22.
- Brugger W, Triller N, Blasinska-Morawiec M, et al.
Prospective molecular marker analyses of EGFR and
KRAS from a randomized, placebo-controlled study of
erlotinib maintenance therapy in advanced non-small-cell
lung cancer. J Clin Oncol 2011;29:4113-20.
- Tran HT, Zinner RG, Blumenschein GR Jr, et al.
Pharmacokinetic study of the phase III, randomized,
double-blind, multicenter trial (TRIBUTE) of paclitaxel
and carboplatin combined with erlotinib or placebo in
patients with advanced Non-small Cell Lung Cancer
(NSCLC). Invest New Drugs 2011;29:499-505.
- Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase
III study of erlotinib in combination with cisplatin and
gemcitabine in advanced non-small-cell lung cancer: the
Tarceva Lung Cancer Investigation Trial. J Clin Oncol
2007;25:1545-52.
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE:
a phase III trial of erlotinib hydrochloride (OSI-774)
combined with carboplatin and paclitaxel chemotherapy
in advanced non-small-cell lung cancer. J Clin Oncol
2005;23:5892-9.
- Lee S, Rudd R, Khan I, et al. TOPICAL: Randomized
phase III trial of erlotinib compared with placebo in
chemotherapy-naive patients with advanced non-small
cell lung cancer (NSCLC) and unsuitable for first-line
chemotherapy. J Clin Oncol 2010;28:15s (suppl; abstr 7504).
- Lilenbaum R, Axelrod R, Thomas S, et al. Randomized
phase II trial of erlotinib or standard chemotherapy in
patients with advanced non-small-cell lung cancer and a
performance status of 2. J Clin Oncol 2008;26:863-9.
- Reck M, Von Pawel J, Fischer JR, et al. Erlotinib versus carboplatin/vinorelbine in elderly patients (age 70 or older)
with advanced non-small cell lung carcinoma (NSCLC):
A randomized phase II study of the German Thoracic
Oncology Working Group. J Clin Oncol 2010;28:15s
(abstr 7565).
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib
versus standard chemotherapy as first-line treatment for
European patients with advanced EGFR mutation-positive
non-small-cell lung cancer (EURTAC): a multicentre,
open-label, randomised phase 3 trial. Lancet Oncol
2012;13:239-46.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus
chemotherapy as first-line treatment for patients with
advanced EGFR mutation-positive non-small-cell lung
cancer (OPTIMAL, CTONG-0802): a multicentre,
open-label, randomised, phase 3 study. Lancet Oncol
2011;12:735-42.
- Gridelli C, Morgillo F, Favaretto A, et al. Sorafenib in
combination with erlotinib or with gemcitabine in elderly
patients with advanced non-small-cell lung cancer: a
randomized phase II study. Ann Oncol 2011;22:1528-34.
- Ym C, Cm T, Wc F, et al. Phase II randomized trial of
erlotinib or vinorelbine in chemonaive, advanced, nonsmall
cell lung cancer patients aged 70 years or older. J
Thorac Oncol 2012;7:412-8.
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as
maintenance treatment in advanced non-small-cell lung
cancer: a multicentre, randomised, placebo-controlled
phase 3 study. Lancet Oncol 2010;11:521-9.
- Miller Va, O’connor P, Soh C, et al. A randomized,
double-blind, placebo-controlled, phase IIIb trial (ATLAS)
comparing bevacizumab (B) therapy with or without
erlotinib (E) after completion of chemotherapy with B
for first-line treatment of locally advanced, recurrent, or
metastatic non-small cell lung cancer (NSCLC). J Clin
Oncol 2009;27:18s (abstr LBA8002).
- Mok Ts, Wu YL, Yu CJ, et al. Randomized, placebocontrolled,
phase II study of sequential erlotinib and
chemotherapy as first-line treatment for advanced nonsmall-
cell lung cancer. J Clin Oncol 2009;27:5080-7.
- Perol M, Chouaid C, Milleron BJ, et al. Maintenance
with either gemcitabine or erlotinib versus observation
with predefined second-line treatment after cisplatingemcitabine
induction chemotherapy in advanced
NSCLC: IFCT-GFPC 0502 phase III study. J Clin Oncol
2010;28:15s (suppl; abstr 7507).
- Herbst RS, O’neill VJ, Fehrenbacher L, et al. Phase
II study of efficacy and safety of bevacizumab in
combination with chemotherapy or erlotinib compared
with chemotherapy alone for treatment of recurrent or
refractory non small-cell lung cancer. 41. Vamvakas L,
Agelaki S, Kentepozidis Nk, Karampeazis A, Pallis Ag and
Christophyllakis C. Pemetrexed (MTA) compared with
erlotinib (ERL) in pretreated patients with advanced nonsmall
cell lung cancer (NSCLC): Results of a randomized
phase III Hellenic Oncology Research Group trial [J]. J
Clin Oncol 2010; 28: 15s (suppl; abstr 7519).
- Vamvakas L, Agelaki S, Kentepozidis Nk, et al.
Pemetrexed (MTA) compared with erlotinib (ERL) in
pretreated patients with advanced non-small cell lung
cancer (NSCLC): Results of a randomized phase III
Hellenic Oncology Research Group trial [J]. J Clin Oncol
2010; 28: 15s (abstr 7519).
- Natale RB, Thongprasert S, Greco FA, et al. Phase III trial
of vandetanib compared with erlotinib in patients with
previously treated advanced non-small-cell lung cancer. J
Clin Oncol 2010;29:1059-66.
- Boyer Mj, Blackhall Fh, Park K, Barrios Ch, Krzakowski
Mj and Taylor I. Efficacy and safety of PF299804 versus
erlotinib (E): A global, randomized phase II trial in patients
(pts) with advanced non-small cell lung cancer (NSCLC)
after failure of chemotherapy (CT) [J]. J Clin Oncol 2010;
28: 18s (abstr LBA7523).
- Kim ST, Uhm JE, Lee J, et al. Randomized phase II study
of gefitinib versus erlotinib in patients with advanced nonsmall
cell lung cancer who failed previous chemotherapy.
Lung Cancer 2012;75:82-8.
- Clarke MJ, Stewart LA. Obtaining data from randomised
controlled trials: how much do we need for reliable and
informative meta-analyses? BMJ 1994;309:1007-10.
- Bria E, Gralla RJ, Raftopoulos H, et al. Comparing two
methods of meta-analysis in clinical research - individual
patient data-based (IPD) and literature-based abstracted
data (AD) methods: Analyzing five oncology issues
involving more than 10,000 patients in randomized clinical
trials (RCTs). J Clin Oncol 2007;25:s6512.
|