
Neoadjuvant treatment of stage IIIA-N2 in EGFR-Mutant/ALK-rearranged non-small cell lung cancer
Introduction
The molecular testing of oncogenic drivers with the potential for target inhibition, such as epidermal growth factor receptor (EGFR) and BRAF mutations, as well as rearrangements involving the anaplastic lymphoma kinase (ALK) and ROS1 gene, is endorsed by several guidelines and considered mandatory at diagnosis only in advanced-stage non-small cell lung cancer (NSCLC) (1,2). These alterations are considered powerful predictive biomarkers and constitute excellent therapeutic targets in advanced NSCLC patients.
EGFR mutations constitute the second most common oncogenic driver, after the Kristen Rat Sarcoma viral oncogene (KRAS). Unfortunately, KRAS mutations have not been targeted successfully similar to EGFR mutations (3). Currently, there are 3 generations of tyrosine kinase inhibitors (TKIs) approved in the first-line treatment of patients with advanced NSCLC harboring EGFR-sensitive mutations at exon19 (Ex19del) and substitutions in exon 21 (L858R). Trials with first- (erlotinib and gefitinib), second- (afatinib, dacomitinib) and third-generation (osimertinib) EGFR-TKIs—the latter specifically designed to selectively inhibit the resistant EGFR-T790M mutation—have demonstrated a reduction in the risk of progression in patients with EGFR mutations (4,5). The substantial OS improvement seen with first-line osimertinib in the FLAURA trial (6), with a median overall survival (OS) of 38.6 months compared to 31.8 months with first-generation TKIs, in addition to the favorable tolerability profile, has placed osimertinib as one of the preferred options for initial therapy in patients with EGFR-mutated (EGFR+) advanced NSCLC.
Conversely, ALK-rearranged (ALK+) adenocarcinoma constitutes the second most common subset of targetable oncogenic alterations in advanced non-squamous NSCLC. Crizotinib, a first-generation ALK-inhibitor, was the first targeted drug to reach approval in ALK+ disease after a phase III trial demonstrated its superiority over chemotherapy (7). Since then, second-generation ALK-TKIs—alectinib, ceritinib and brigatinib—have demonstrated greater activity and have been incorporated into the therapeutic arsenal of ALK+ advanced NSCLC patients (8-11). All of these compounds have prolonged median OS times of about 7 years in patients with stage IV ALK+ NSCLC, compared with 4 months among these patients before these drugs were available. More potent third-generation ALK-TKIs such as Lorlatinib—approved for the treatment of patients with ALK+ and progressive disease following treatment with two other prior ALK-TKIs (12)—or ensartinib are currently being studied in treatment-naïve ALK+ patients to see whether they can improve the survival advantage observed with the newest-generation ALK-TKIs.
Despite the encouraging results in treating EGFR/ALK+ driven tumors at advanced stages, the clinical value of using targeted inhibitors in an early-stage context, within surgery, has not yet been defined. Indeed, there has been little improvement in the management of resectable NSCLC in nearly a decade. To date, guideline recommendations for surgically resectable patients with oncogenic drivers are the same as for those without driver alterations, and following surgical resection, adjuvant platinum-based chemotherapy remains the mainstay treatment (13).
In this review, we aim to provide a comprehensive overview of current knowledge advances about this distinct biologic subgroup of NSCLC patients with alterations in EGFR and ALK but focusing on early-stage and locoregional (N2) disease. We will put the epidemiology and prognostic significance of these two molecular entities into context and we will discuss the opportunity to maximize progress towards better outcomes by overviewing the results of the most relevant clinical trials exploring the use of induction/adjuvant targeted therapies at earlier stages of the disease. We present the following article in accordance with the narrative review reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-780).
Methods
We have performed an exhaustive and systematic literature search using public, published citations for biomedical literature on PubMed, databases of registered clinical trials for unpublished results, and abstracts presented at international conferences.
Occurrence of EGFR/ALK alterations in early-stage and locoregional (N2) lung cancer
There is no clear data about the real frequency and type of EGFR mutations and ALK translocations in early-stage or locoregional (N2) disease, as standard biomarker testing in this setting is currently not endorsed by molecular guidelines (13).
Several retrospective studies have reported varying EGFR mutation rates, ranging between 17% to 20% in non-Asian populations (14,15) and 28% to 54% in Asian populations (16,17). In a large-scale Chinese retrospective analysis of 790 early-stage resected tumors, the EGFR mutation rate and mutation type were very similar to those in the advanced-stage (53.6% vs. 51.4%) with an incidence comparable among diverse nodal stages (N0: 55.2%, N1: 45.5%, N2: 44.8%) (16). In the ADAURA trial (NCT02511106), a phase III trial assessing the efficacy and safety of adjuvant osimertinib in resected stage IB–IIIA EGFR+ NSCLC, a total of 2447 resected specimens (61% Asian patients) were screened, for which 44% were EGFR+, with a higher proportion of Asian vs. non-Asian (63% vs. 37%), and female vs. male (69% vs. 30%) (18,19). The high incidence of EGFR mutations assigned to the early-stage disease is confounding, and might reflect a selection bias result of the retrospective nature of some cohort studies and the pre-screening process of potential subjects to determine their initial eligibility in others.
The Lungscape Project, a European multi-institutional consortium biobank from the European Thoracic Oncology Platform (ETOP), explored the incidence of gene alterations in a large cohort of surgically resected stage I–III NSCLC (20,21). The estimated prevalence of EGFR mutations was 5.4% (9.7% in adenocarcinomas) (20) and 6.2% and at least 2.2% by either immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) for ALK+, respectively (21). Other recent retrospective cohort studies have reported similar rates for early-stage ALK+ NSCLC (22).
Prognostic significance and clinical outcomes of EGFR/ALK alterations in early-stage lung cancer
Based on the revised eighth edition of the TNM staging system, the 5-year OS for early-stage NSCLC by best-stage groups (pathological if available, otherwise clinical stage) ranges from 89% to 71% for stage I, 64% to 55% for stage II, and 37% for stage IIIA (23). More specifically, for one single-category N2, disease survival rates are much poorer with median OS of 17 months and only 23% of patients alive at 5 years (23). However, the prognosis of the N2 descriptor strongly differs between the microscopic N2 involvement and the bulky disease with perinodal infiltration (24,25).
To date, defining the prognostic impact of molecular drivers of early-stage and locoregional (N2) disease has been challenging and inconsistent due to the relative rarity of these subsets. Additionally, it is unknown whether these specific molecular alterations might influence the natural history of surgically resected NSCLC. Some retrospective cohorts have shown a significant survival advantage in surgically resected EGFR+ compared with EGFR wild-type patients (14,26). However, others could not confirm the prognostic significance of EGFR mutational status (20,27). A large meta-analysis which included a large dataset of 9,635 surgically-resected NSCLC patients pooled from 32 studies, shown a significant advantage in disease-free survival (DFS) (HR 0.77; 95% CI, 0.66–0.90; P=0.001) and OS (HR 0.72; 95% CI, 0.66–0.80; P<0.00001) in early-stage EGFR+ NSCLC (28). Putting the data together, these findings might indicate that surgically treated NSCLC patients with EGFR mutations could be inclined to exhibit better outcomes after surgery. It cannot be excluded, however, that the better outcomes result from other frequently associated good prognostic factors, such as younger age, female sex, or non-smoking habits.
The prognostic significance of ALK positivity in early-stage disease is also debatable. Whereas some studies suggest that ALK positivity confers worse prognostic outcomes (22,29-31), other studies and meta-analyses have proven a favorable prognostic association in patients with surgically resected NSCLC (21,32).
The significance of the genotype status was also evaluated in a large cohort of locally-advanced NSCLC patients treated with conventional radiotherapy and chemotherapy with curative intent (57% stage IIIA) (33). Median OS was significantly higher for EGFR+ and ALK+ compared to KRAS+ and wild-type patients (55.8 vs. not reached vs. 28.0 vs. 33.2 months respectively; P=0.02), with lower incidence of locoregional recurrence in EGFR+ patients (3-year estimate freedom of recurrence: 77% vs. 38% vs. 49% vs. 46% respectively; P=0.08). No differences in the risk of distant metastases was observed among the subgroups (33).
Neoadjuvant strategies in locoregional (N2) lung cancer and surrogate pathological endpoints
The locoregional N2 disease (stage IIIA) accounts for approximately 15% of NSCLC cases (34). The optimal treatment modalities for patients with N2 disease remains a matter of debate in thoracic oncology (35) and the definition of ‘resectability’ for N2 disease differs considerably between countries and centers (36,37). For patients with ‘unresectable’ N2 disease, the standard of care is less debatable, as concurrent chemo-radiotherapy followed by maintenance with durvalumab is recommended if no evidence of disease progression is observed after the induction treatment (PACIFIC trial) (38). In potentially resectable stage IIIA (N2) disease—patients in whom a microscopically margin-negative resection may be anticipated—diverging trimodality approaches including surgery, perioperative chemotherapy, and radiotherapy may be considered within the framework of a multidisciplinary thoracic team (13).
Neoadjuvant chemotherapy has been shown to be a valid alternative to adjuvant chemotherapy in early-stage IB-IIIA NSCLC, with a 13% reduction in the relative risk of death (HR 0.87; 95% CI, 0.78–0.96; P=0.007) and an absolute 5-year benefit of 5% in overall survival and 10% in time-to-distant recurrence (39). More specifically, for stage IIIA (N2) NSCLC, several randomized-controlled trials and meta-analyses have also demonstrated a substantial survival advantage with the use of neoadjuvant chemotherapy (40,41). Preoperative chemoradiotherapy is an alternative option to neoadjuvant chemotherapy in patients with stage IIIA(N2) NSCLC, and it increases the proportion of complete resections (75% vs. 60%) as well as the rate of mediastinal downstaging (46% vs. 29%, P=0.02) and pathological responses (60% vs. 20%, P<0.0001). However, both treatment strategies seem to provide similar benefits in terms of PFS and OS (42).
Despite all of the controversies over which is the ‘optimal’ treatment approach for N2 disease, the early introduction of a systemic treatment in patients suitable for surgical resection seems to be a reasonable approach and poses several advantages: (I) improves treatment compliance; (II) enhances local control increasing the likelihood of a complete resection; (III) has the potential to eradicate subclinical metastases; (IV) allows an in vivo assessment of the biological changes in the tumor at resection and an early readout of efficacy, constituting a more accurate means of comparing the activity of novel neoadjuvant therapies within a few weeks from treatment initiation and last but not least; (V) a patient’s response to neoadjuvant chemotherapy may provide early prognostic information on tumor response and downstaging.
Collectively, several trials have demonstrated a prognostic association of mediastinal pathological lymph node downstaging (pLND), pathologic responses and complete tumor resection (R0) in locoregional stage IIIA (pN2 by mediastinoscopy) following both neoadjuvant chemotherapy and chemoradiation (43-46).
Complete pathological response (CPR)—defined as the absence of viable tumor cells in resected specimens—is an accepted surrogate measure for survival in patients with breast cancer following completion of neoadjuvant systemic therapy, and supports an accelerated drug approval by the Food and Drug Administration (FDA) (47). However, in lung cancer no pathologic surrogate following neoadjuvant therapy has been broadly accepted or defined so far. In lung cancer, CPR after neoadjuvant chemotherapy is rather infrequent (about 4–10%) and the utility of mediastinal downstaging is limited as it depends on the accuracy of nodal assessment at diagnosis (48). Recently, the concept of major pathologic response (MPR), which is defined as 10% or less residual tumor cells after neoadjuvant therapy, has been proposed as a potential surrogate endpoint for OS in patients with NSCLC and a conceivable marker to evaluate the response of novel therapies in biomarker-driven translational clinical trials (48,49).
In light of the growing need, the International Association of Lung Cancer (IASLC) has launched a multidisciplinary recommendation for the pathologic assessment of lung cancer resection specimens after neoadjuvant therapy to define pathologic response, including MPR or CPR, after neoadjuvant therapy in clinical trials and in routine practice (50). In order to facilitate the comparison of the impact of pathologic responses between different types of neoadjuvant therapies, the guidelines recommend using the same approach be used when evaluating the percentage of viable tumor cells of resected lung cancers regardless of the type of neoadjuvant therapy administered, including molecularly targeted or immunological therapies as well as combination strategies. Whether these pathological responses can be used as surrogate endpoints in lung cancer patients treated with neoadjuvant targeted therapies is presently unknown but merits further evaluation in prospective trials.
The role of targeted therapies in the perioperative management of resectable EGFR/ALK+ NSCLC
Adjuvant targeted trials in EGFR+ NSCLC
Platinum based chemotherapy has been the mainstay treatment in the adjuvant setting of NSCLC for about two decades, offering a modest 4–5% absolute benefit at 5 years for patients with stage II and III (N1 and N2 disease) (51), and the use of adjuvant targeted therapies is not yet approved in early-stage NSCLC. Given the demonstrated efficacy of several EGFR-TKI in advanced disease, it was reasonable to postulate that perioperative targeted therapies might also impact the outcomes of EGFR-mutant NSCLC patients at earlier stages. Indeed, the use of targeted therapies as adjuvant treatment of oncogene-driven cancers is well established in other diseases such as pertuzumab or trastuzumab in HER2-positive breast cancer, dabrafenib/trametinib in BRAF-mutated melanoma, or imatinib in KIT-mutant gastrointestinal stromal tumors (52-55). On this basis, several studies have been designed to investigate the role of EGFR-TKIs in the adjuvant setting of EGFR+ NSCLC (Table 1).

Full table
The National Cancer Institute of Canada (NCIC) BR19 trial (61), was the first randomized, placebo-controlled trial of an EGFR-targeted agent (gefitinib, administered for two years) delivered in the adjuvant setting in completely resected stage NSCLC (IB-IIIA). When BR19 was initiated, it was unknown that activating mutations of EGFR were biomarkers of efficacy for EGFR-TKI and patients were unselected for the EGFR+ genotype. The trial was closed prematurely after an interim analysis showed that maintenance gefitinib was associated with worse outcomes than placebo (OS HR, 1.24; 95% CI, 0.94–1.64; P=0.14; DFS HR, 1.22; 95% CI, 0.93–1.61; P=0.15). The molecular profile was determined as an exploratory analysis in 71% of tumors (359/503). For the subset of EGFR mutation-positive tumors, gefitinib also did not demonstrate a beneficial effect on outcomes, although the interpretation of the data is limited by the low number of patients included (n=15).
The second randomized, double-blind, phase III trial for postoperative EGFR-TKI was the RADIANT trial (62). Patients with completely resected stage IB to IIIA NSCLC (n=973) whose tumors expressed EGFR protein (IHC) or EGFR amplification (FISH) were randomized to receive erlotinib or a placebo for two years. Adjuvant erlotinib did not prolong disease-free survival (DFS) over placebo in patients with EGFR-expressing NSCLC (HR 0.90; 95% CI, 0.74–1.10; P=0.324). Secondary endpoints included the evaluation of DFS and OS in patients with EGFR+ (ex19del/L858R). Although an initial favorable DFS with erlotinib was observed among the 161 patients (16.5%) with EGFR-mutations (median 46.4 vs. 28.5 months; HR 0.61; 95% CI, 0.38–0.98; P=0.039), with a longer follow-up at 60 months, this trend of erlotinib benefit in the EGFR+ subgroup was no longer apparent (HR 0.75; 95% CI, 0.48–1.15; P=0.19) (63).
Unlike the BR19 and the RADIANT trials, the SELECT trial evaluated the role of adjuvant EGFR-TKIs specifically in molecularly selected EGFR+ NSCLC (57). In this phase II study, one hundred patients with resected stage IA to IIIA and sensitive EGFR mutations (3 patients with uncommon mutations G719X and L861Q), were treated with erlotinib for 2 years after standard adjuvant chemotherapy with or without radiotherapy. Most patients (69%) consented to completing up to 2 years of therapy. The study met its primary endpoint of improving the 2-year DFS (overall 88%; 96% stage I; 78% stage II; 91% stage III) compared to the historical genotype-matched controls (76%). At 5-years, DFS and OS were 56% and 86%, respectively, with medians that have not yet been reached. Recurrences occurred in 40 of patients, mainly in those who received a significantly shorter duration of adjuvant erlotinib, and patients rechallenged with erlotinib (n=26) experienced durable benefit (median 13.1 months) (57). In spite of the promising overall results, this was a single-arm, phase II study so efficacies could not be used for direct comparison.
An updated meta-analysis on adjuvant EGFR-TKIs (seven trials including the BR19, RADIANT and ADJUVANT trials) provided strengthened evidence of adjuvant EGFR-TKI treatment in the EGFR+ sub-population with a HR for DFS of 0.51 (95% CI, 0.39–0.65), corresponding to an absolute benefit of 7% at 3 years (48.5% vs. 55.6%) (64).
The ADJUVANT-CTONG 1104 trial (65) is the first reported OS result of a phase III randomized trial of adjuvant EGFR-TKI vs. standard chemotherapy in resected stage II–IIIA (N1–N2) EGFR+ NSCLC. One of the study limitations is that there were more patients allocated to the chemotherapy group, who did not receive treatment, than that of the gefitinib group. The study met its primary endpoint of improving DFS by 10 months with adjuvant gefitinib compared with standard doublet chemotherapy (28.7 vs. 18.0 months; HR 0.60; 95% CI, 0.42–0.87, P=0.0054). A subgroup analysis found that N2 patients retained a higher benefit in DFS (HR 0.52; 95% CI, 0.34–0.80, P=0.003). The final 3- and 5-year DFS rate and OS have recently been updated with a median follow-up of 80.0 months (56). The slight final improvement in DFS (30.8 vs. 19.8 months; HR 0.56; 95% CI, 0.40–0.79) could not translate into a significant OS difference, and final median OS did not differ between groups (75.5 months in gefitinib group and 62.8 months in control group; HR 0.92; 95% CI, 0.62–1.36). Moreover, after 3 years of follow-up, the curves came together and the benefits initially achieved were no longer observed (3-year DFS 39.6% vs. 32.5%). These unfavorable results raise the possibility that some patients may have had extended disease at the time of enrollment. Indeed, the trial enrolled a large percentage of patients with stage IIIA disease (64%) and did not require fluorodeoxyglucose-positron emission tomography (FDG-PET) scans at baseline for staging.
Two Asian trials explored the use of adjuvant EGFR-TKIs specifically in resected locoregional stage IIIA (N2) NSCLC. The phase 2 EVAN trial, compared erlotinib for 2 years vs. four cycles of platinum-based chemotherapy in 102 resected stage IIIA EGFR+ NSCLC patients from China. The trial met its primary endpoint and confirmed that adjuvant erlotinib was superior to cisplatin-based chemotherapy, with an absolute 2-year DFS gain of 36.7% (HR 0.268; 95% CI, 0.13–0.53, P<0.0001), an absolute gain that was retained with longer follow up at 3-years (34.4%) (58). It is worth highlighting that 94% of all patients (48/51) were stage IIIAN2 and received the most benefit from targeted therapy, which is consistent with the ADJUVANT trial results. The OS of the EVAN trial remains immature.
The consolidation strategy after chemotherapy using a shorter maintenance continuation of EGFR-TKI of about 8 and 6 months has been explored in two small phase II randomized trials (59,60) (Table 1). In one of these trials, patients with resected stage IIIA–N2 NSCLC harboring EGFR mutations were assigned to receive chemotherapy for 4 cycles, followed with or without gefitinib (250 mg/day), for 6 months. DFS was significantly longer among those who received pemetrexed-carboplatin (PC) and gefitinib than among those who received PC alone (median, 39.8 vs. 27.0 months; HR 0.37; 95% CI, 0.16–0.85; P=0.014). The rates of 2-year DFS were 78.9% in the PC-gefitinib group and 54.2% in the PC alone group.
The ADAURA trial (NCT02511106), is the first randomized phase III trial which has explored a third generation EGFR-TKI (osimertinib) as adjuvant therapy in patients with EGFR+ NSCLC. The ADAURA is a double-blind, randomized, placebo-controlled trial comparing the efficacy of osimertinib 80 mg daily or placebo for three years following complete tumor resection in 700 completely resected EGFR-mutant stage IB–IIIA NSCLC patients after adjuvant chemotherapy. One third of the patients included in the study were stage IIIA and 61% Asian. The primary efficacy endpoint was DFS by investigator assessment in stage II/IIIA patients, designed for superiority under the assumed DFS HR of 0.70. Following an independent committee recommendation, the study was unblinded early due to efficacy, and the results of the unplanned interim analysis have recently been reported with a follow-up of at least 1 year (19). The study met its primary endpoint and showed a statistically significant and clinically meaningful improvement in DFS in patients with stage II–IIIA EGFR+ NSCLC treated with osimertinib vs. placebo (median NR vs. 20.4; HR 0.17; 95% CI, 0.12–0.23; P<0.0001). Overall (stage IB–IIIA), there was a 79% reduction in the risk of disease recurrence or death with osimertinib vs. placebo (HR 0.21; 95% CI, 0.16–0.28; P<0.0001) and 2-year DFS rates were 89% vs. 53%, respectively, with curves that separated early and did not cross over time. The benefit of DFS was observed in all of the prespecified subgroups regardless of whether patients had received prior adjuvant chemotherapy and was high and consistent among all of the different stages (overall HR for stage IB, II, and IIIA were 0.5, 0.17, and 0.12, respectively). We do not know whether the use of a third-generation more potent inhibitor (osimertinib) or the longer exposition to the inhibitor (3 years) might explain the improved results. The safety profile was consistent with the established safety profile of osimertinib, with only mild EGFR-TKI class effects reported [Grade ≥3 adverse events (AEs) 20%]. At the time of analysis, median OS was still immature to draw any conclusions.
Some of the abovementioned adjuvant clinical trials, stratified the patients by EGFR mutation status (Ex19del or L858R) in order to perform subgroup analysis. This is the case of the adjuvant phase III ADAURA and ADJUVANT/CTONG1104 trials and the phase II EVAN trial. In the Li et al. trial analysis of DFS by mutation subtype was preplanned. All these studies, consistently agree that patients harboring the exon 19 deletion seem to derive a major benefit to TKIs than patients with L858R in terms of DFS. On the other hand, whether the duration of adjuvant TKI therapy might result in distinct efficacy remains unknown and there is a need for further investigation. Two years of therapy was selected in the CTONG1104, SELECT, and EVAN trials, whereas 3 years of osimertinib was used in the ADAURA trial. The median duration of exposure to adjuvant erlotinib or osimertinib in the EVAN, SELECT, and ADAURA trials was very consistent (23.9, 20.0, and 22 months, respectively), whereas a lower gefitinib exposure was reported in RADIANT (11.9 months). In the ADJUVANT-CTONG 1104 trial (26), patients with longer exposure to adjuvant gefitinib for ≥18 months seemed to retain a higher OS benefit (HR 0.38; 95% CI, 0.22–0.66; P<0.001).
There are four phase III trials ongoing in the adjuvant setting for resected stage II-IIIA NSCLC: the Chinese ICTAN (NCT01996098), ICWIP (NCT02125240) and EVIDENCE (NCT02448797) with adjuvant icotinib and the Japanese IMPACT (WJOG6401L) with gefitinib (Table 2).
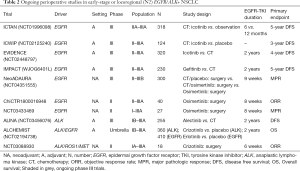
Full table
Neoadjuvant targeted trials in EGFR+ NSCLC
Unlike the adjuvant context, only phase II trials with a very limited number of patients have evaluated the role of induction targeted TKI therapies in EGFR+ resectable NSCLC (Table 3).

Full table
There is evidence of the feasibility and activity of using neoadjuvant targeted modalities in other oncogenic-depend tumors, such as pertuzumab and trastuzumab in HER2-positive breast cancer (71,72).
The CSLC-0702 was the first phase II trial of neoadjuvant treatment stratified by EGFR mutation status (66). Twenty-four patients with stage IIIA (N2) NSCLC were randomized according to the status of EGFR to receive three cycles of gemcitabine plus carboplatin (EGFR-wild type) or erlotinib (EGFR+) during 42 days before the surgery. Although the neoadjuvant treatment with erlotinib in EGFR+ tended to show an improved response rate (58.3% vs. 25%), the PFS and OS did not differ significantly between two arms and only 50% (3/6) EGFR+ patients underwent surgical resection compared to 71% (5/7) in the chemotherapy arm. However, results should be taken with caution due to the small number of patients included in the trial.
Zhong et al. (67) compared the efficacy of erlotinib vs. gemcitabine plus cisplatin as neoadjuvant/adjuvant treatment in 72 Chinese patients with stage IIIA (N2) NSCLC molecularly selected for sensitizing EGFR mutations in exon 19 or 21 (CTONG1103-EMERGING trial). Patients received erlotinib 150 mg/day (neoadjuvant therapy, 42 days; adjuvant therapy, up to 12 months) or gemcitabine plus cisplatin (neoadjuvant therapy, two cycles; adjuvant therapy, up to two cycles). The primary endpoint of ORR was not met for neoadjuvant erlotinib vs. chemotherapy (ORR 54.1% vs. 34.3%, OR 2.26; 95% CI, 0.87–5.84; P=0.092) and no CPR were identified in either arm. Even so, an MPR rate of 9.7% with erlotinib vs. 0% with chemotherapy was demonstrated, with a pLND reported in 11% (4/37) in the erlotinib group and 3% (1/35) in the chemotherapy group. After neoadjuvant therapy, 73% of patients in the erlotinib group and 63% in the chemotherapy group underwent surgery. Median PFS was significantly longer with erlotinib versus chemotherapy (21.5 vs. 11.4 months; HR 0.39; 95% CI, 0.23–0.67; P=0.001) but OS was not significantly different between the two arms (45.8 vs. 39.2 months; HR 0.77; 95% CI, 0.41–1.45; P=0.417).
The ESTERN trial (68), was a small (n=19), single-arm study, evaluating whether neoadjuvant erlotinib at standard dose of 150 mg daily for 56 days could improve operability and OS in patients with stage IIIA (N2) EGFR+ NSCLC. The radical resection rate (RRR) was 68.4% (14/19), 35.7% of patients (5/14) achieved a pLND following treatment, with an ORR of 42.1%. Among the 19 patients who received neoadjuvant therapy, median PFS and OS were 11.2 and 51.6 months, respectively.
Zhang and colleagues conducted a single-arm, small (n=35) phase II trial (NCT01833572) to evaluate the efficacy of gefitinib (250 mg once daily for 42 days) as neoadjuvant treatment in patients with stage II–IIIA EGFR+ NSCLC (69). The ORR, primary endpoint, was 54.5% and the rate of MPR was 24.2%. The median DFS was 33.5 months (95% CI, 19.7–47.3), whereas median OS was not reached.
Another ongoing study evaluating a pre-operative strategy with gefitinib for resectable IA–IIIA EGFR+ NSCLC is the PROGRESS trial (NCT02804776) (70). The primary endpoint was to determine EGFR-TKI sensitivity biomarkers in responders vs. non-responders, correlating pathologic responses with serial plasma and tissue sequencing, and pharmacodynamic changes with serial FDG-PET scans. Results were recently presented about sixteen patients who received a minimum of 4 weeks gefitinib 250 mg once daily. The ORR was 62%, all patients underwent resection, 31% (4/13) had pLND and 8% (1/13) had an MPR. The percentage of residual disease did not correlate with residual FDG-uptake or tumor response. Interestingly, by RNA-sequencing, there was a prevalent upregulation of immune regulatory and inflammatory response genes, indicating infiltration of fibroblasts and T cells, providing unique insight into adaptive responses and for the development of rational combination approaches in EGFR+ NSCLC.
The role of the newest generation EGFR-TKIs such as osimertinib as a neoadjuvant therapy in early-stage EGFR+ NSCLC has not been established. To date, there are three ongoing clinical trials exploring the efficacy of osimertinib in a neoadjuvant setting: the phase II ChiCTR1800016948 and NCT03433469, and the phase III NeoADAURA (NCT04351555) (Table 2). Preliminary data from the phase II NCT03433469 study (comprising data from only 5 patients) indicates that 8 weeks of neoadjuvant treatment with osimertinib is well tolerated (no serious AEs reported) with a 60% ORR (no CPR have been observed) (73). The NeoADAURA trial, will randomize (1:1:1) 300 patients with sensitizing EGFR mutations (Ex19del or L858R either alone or in combination with other EGFR mutations) to receive placebo plus chemotherapy (per 3 cycles), osimertinib plus chemotherapy (per 3 cycles) and osimertinib (9 weeks), duration a bit longer than previous neoadjuvant trials with EGFR-TKIs.
Perioperative targeted trials in ALK+ NSCLC
In early-stage ALK+ disease, there is no available information on the role of ALK-TKIs in the adjuvant setting and only two phase III trials are currently recruiting participants (Table 2).
The ALINA (NCT03456076) is the only phase III ongoing trial, investigating the efficacy of adjuvant ALK-TKI alectinib in resected stage IB-IIIA ALK+ NSCLC. A total of 255 patients will be randomized (1:1) to receive twice-daily alectinib 600 mg for 2 years or four 21-day cycles of platinum-based chemotherapy. The primary endpoint of the study is DFS per investigator and secondary endpoints include OS, safety, and pharmacokinetics. The ALCHEMIST (NCT02194738) is another prospective, randomized, double-blind, placebo-controlled trial including stage IB-IIIA patients with EGFR+ or ALK rearrangements after surgical resection. The umbrella design of the study facilitates targeting several oncogenic-drivers at a time to subsequently refer them to one of the treatment trials—the ALCHEMIST-EGFR (A081105) or ALCHEMIST-ALK (E4512)—which are testing erlotinib (for EGFR+) or crizotinib (for ALK+) versus observation (74). The primary objective of the trial is to evaluate whether drug treatments targeted against those molecular changes can lead to an improved survival.
Likewise, in the neoadjuvant setting, we do also have very marginal evidence coming from two small studies and there is only one small phase II trial (NCT03088930) currently ongoing evaluating the efficacy of crizotinib (6 weeks as an induction therapy) in 18 patients with resectable stage IA-IIIA NSCLC (Table 2).
A retrospective case cohort with small sample size (n=11) reported the efficacy of neoadjuvant crizotinib (250 mg twice daily for a median duration of 30 days) in ALK+ patients with pathologically confirmed N2 disease (75). All patients showed promising response to induction treatment allowing for surgery, and two patients (18.2%) attained a CPR. Five patients with disease recurrence were treated with crizotinib achieving a long duration of response. The phase II SAKULA (UMIN000017906), is a Japanese trial that evaluated the efficacy and safety of neoadjuvant ceritinib (750 mg once daily for 12 weeks) followed by surgery in patients with ALK+ stage II–III NSCLC (76). Due to slow accrual, only seven patients were finally enrolled (all them had stage IIIA disease). The reported ORR was 100% and surgical resection was performed in six patients. MPR (the primary endpoint of the study) and CPR were 57% and 33% respectively. With a median follow-up of 10 months, one patient died of disease progression and six patients remain alive, including four patients who are recurrence-free (76).
Other strategies beyond targeted therapies in early-stage EGFR/ALK+ NSCLC
Preliminary results of neoadjuvant immunotherapy in NSCLC are encouraging with CPR and MPR of about 71% and 85% respectively (77), significantly higher compared with the historical CPR rates of 4–10% reported with neoadjuvant chemotherapy or chemoradiation (48).
However, the place of immunotherapy in patients with oncogenic drivers is questioned. In patients with EGFR- and ALK actionable alterations, a low response rate with the use of immune checkpoint inhibitors (ICI) as a single agent has been reported in advanced disease. In a retrospective, multi-center study for patients with at least one oncogenic driver alteration receiving ICI monotherapy (IMMUNOTARGET registry), the ORR by driver was 12% for EGFR+ and 0% in ALK+ patients with a median PFS of 2.1 months for EGFR+ and 2.5 months for ALK+ patients (78). On the other no additive efficacy but a higher immune-related G3‒5 AEs (including pneumonitis) has been reported with the use of ICI plus TKIs (79,80).
Although there are several studies exploring neoadjuvant strategies with immunotherapy, either as monotherapy or in combination with chemotherapy (81), in the vast majority of trials, patients with EGFR/ALK alterations are excluded. Two trials with neoadjuvant atezolizumab as monotherapy or in combination with chemotherapy, evaluated the pathological response in EGFR+ and ALK+ patients. In the LCMC3 trial (NCT02927301) of neoadjuvant atezolizumab, five patients had known driver mutations (4 EGFR+, 1 ALK+) and were excluded per protocol in the efficacy-evaluable population. Data on three of these five patients is available: one patient EGFR+ was no longer resectable and the other two cases had viable tumor cells at the time of surgery (90% in EGFR+ and 60% in ALK+) (82). The IMpower030 trial, atezolizumab plus chemotherapy given as neoadjuvant therapy identified four patients (31%) with EGFR mutations (83). Two of them (L858R and L858R/S768I mutations) achieved CPR, and the other two (ex20Ins and ex19del) did not have an MPR.
Durvalumab is another anti-PD-L1 whose effectiveness and safety are being tested, alone or in combination, in the neoadjuvant setting. The AEGEAN trial (84), will allow researchers to include up to 20% of patients with EFGR/ALK alterations; and the NeoCOAST trial (NCT03794544), will not consider the presence of these biomarkers as an exclusion criteria. We have not been able to identify other induction trials with immunotherapy allowing the inclusion of oncogenic-driven tumors. Further studies should be pursued to evaluate the potential of neoadjuvant combination strategies with chemotherapy and immunotherapy in these oncogenic-dependent tumors.
Conclusions
Unlike breast cancer, the use of perioperative targeted therapies in molecular-driven early-stage NSCLC is debatable and results have been inconsistent so far. The rarity of these subsets and the corresponding slow recruitment have lessened the clinical research development and the accessibility to randomized control-blinded studies in early disease. However, the strategy of identifying and selecting patients for the most appropriate therapy according to their molecular profile must be pursued in order to promote the evolution of individualized perioperative multimodal strategies, so as to understand the biological changes of the tumor, as well as to have a broad overview of the genomic landscape in early-stage disease. This is essential in light of the variety of immunotherapeutic options that are looming on the horizon in the perioperative setting.
Although the initial benefits observed with adjuvant first-second generation EGFR-TKIs are discouraging, new generation TKIs have awoken researchers’ interest and we are optimistic that the consistent improvements in DFS rates at different timepoints in the ADAURA trial with osimertinib will translate into an overall survival benefit in the adjuvant setting of EGFR+ NSCLC. However, we will need to wait patiently until the maturation of the OS curves and anticipate for the outcomes of other ongoing phase III trials to know whether adjuvant targeted therapies are curing more oncogenic patients or rather deferring a disease relapse that cannot be eradicated.
There appears to be no appropriate explanations for why responses with neoadjuvant TKIs in early-stage lung cancer are of less magnitude than those observed in patients in metastatic settings. However, a major limitation of the data interpretation with all of these studies is the lack of randomized trials and the small populations of patients. One may question whether the duration of neoadjuvant TKI is optimal or whether a longer exposure time to the targeted therapy could potentially translate into a higher clinical efficacy without impairing the curative intervention. Meanwhile, we also have the challenge of demonstrating that neoadjuvant surrogate endpoints can be relied upon in early lung cancer to predict, or correlate with, clinical outcomes within molecular subgroups in order to accelerate drug development. To search for better results, we also need large-scale randomized-controlled trials with umbrella designs investigating other thought-provoking approaches such as combination strategies with chemo-immunotherapy or chemo-TKIs in this subset of patients with driver alterations.
We must seize the opportunity to explore the value of minimal residual disease by using liquid biopsies as well as multiomics-based assays to identify the predictive characteristics of patients who would most benefit from neoadjuvant targeted therapies to finally bring personalized medicine to an earlier stage of the disease.
Acknowledgments
We would like to acknowledge Kyla M. Juett for improving the use of English in the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mariano Provencio) for the series “Multimodal management of locally advanced N2 non-small cell lung cancer” published in Translational Lung Cancer Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the NARRATIVE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-780
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-780
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-780). The series “Multimodal management of locally advanced N2 non-small cell lung cancer” was commissioned by the editorial office without any funding or sponsorship. NR reports grants from Pfizer and Novartis; personal fees from MSD, BMS, Takeda, Roche, Boehringer Ingelheim, Astra Zeneca, Guardant Health, Novartis, Amgen and Lilly outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 2018;142:321-46. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29 Suppl 4:iv192-237. [Crossref]
- Scheffler M, Ihle MA, Hein R, et al. K-ras Mutation Subtypes in NSCLC and Associated Co-occuring Mutations in Other Oncogenic Pathways. J Thorac Oncol 2019;14:606-16. [Crossref] [PubMed]
- Haaland B, Tan PS, de Castro G, et al. Meta-analysis of first-line therapies in advanced non-Small Cell lung cancer harboring EGFR-activating mutations. J Thorac Oncol 2014;9:805-11. [Crossref] [PubMed]
- Soria JC, Ramalingam SS. Osimertinib in EGFR Mutation-Positive Advanced NSCLC. N Engl J Med 2018;378:1262-3. [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2019;382:41-50. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naïve advanced ALK-positive non-Small Cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-Small Cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-Small Cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-Small Cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- D'Angelo SP, Janjigian YY, Ahye N, et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 2012;7:1815-22. [Crossref] [PubMed]
- Ragusa M, Vannucci J, Ludovini V, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcome in resected non-small cell lung cancer patients. Am J Clin Oncol 2014;37:343-9. [Crossref] [PubMed]
- Pi C, Xu CR, Zhang MF, et al. EGFR mutations in early-stage and advanced-stage lung adenocarcinoma: Analysis based on large-scale data from China. Thorac Cancer 2018;9:814-9. [Crossref] [PubMed]
- Kim IH, Lee IH, Lee JE, et al. Clinical Significance of C-MET Overexpression and Epidermal Growth Factor Receptor Mutation in Platinum-Based Adjuvant Chemotherapy Outcome in Surgically Resected Lung Adenocarcinoma. Ann Surg Oncol 2017;24:770-7. [Crossref] [PubMed]
- Tsuboi M, Herbst RS, John T, et al. Frequency of epidermal growth factor receptor (EGFR) mutations in stage IB–IIIA EGFR mutation positive non-small cell lung cancer (NSCLC) after complete tumour resection. Ann Oncol 2019;30:v589. [Crossref]
- Herbst RS, Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol 2020;38:abstr LBA5.
- Kerr KM, Dafni U, Schulze K, et al. Prevalence and clinical association of gene mutations through multiplex mutation testing in patients with NSCLC: results from the ETOP Lungscape Project. Ann Oncol 2018;29:200-8. [Crossref] [PubMed]
- Blackhall FH, Peters S, Bubendorf L, et al. Prevalence and clinical outcomes for patients with ALK-positive resected stage I to III adenocarcinoma: results from the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol 2014;32:2780-7. [Crossref] [PubMed]
- Shi J, Gu W, Zhao Y, et al. Clinicopathological and Prognostic Significance of EML4-ALK Rearrangement in Patients with Surgically Resected Lung Adenocarcinoma: A Propensity Score Matching Study. Cancer Manag Res 2020;12:589-98. [Crossref] [PubMed]
- Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2017;12:1109-21.
- Legras A, Mordant P, Arame A, et al. Long-Term Survival of Patients With pN2 Lung Cancer According to the Pattern of Lymphatic Spread. Ann Thorac Surg 2014;97:1156-62. [Crossref] [PubMed]
- Garelli E, Renaud S, Falcoz PE, et al. Microscopic N2 disease exhibits a better prognosis in resected non-Small Cell lung cancer. Eur J Cardiothorac Surg 2016;50:322-8. [Crossref] [PubMed]
- Izar B, Sequist L, Lee M, et al. The Impact of EGFR Mutation Status on Outcomes in Patients With Resected Stage I Non-Small Cell Lung Cancers. Ann Thorac Surg 2013;96:962-8. [Crossref] [PubMed]
- Kim YT, Seong YW, Jung YJ, et al. The Presence of Mutations in Epidermal Growth Factor Receptor Gene Is Not a Prognostic Factor for Long-Term Outcome after Surgical Resection of Non–Small Cell Lung Cancer. J Thorac Oncol 2013;8:171-8. [Crossref] [PubMed]
- Zhang SM, Zhu QG, Ding XX, et al. Prognostic value of. Cancer Manag Res 2018;10:3393-404. [Crossref] [PubMed]
- Yang P, Kulig K, Boland JM, et al. Worse disease-free survival in never-smokers with ALK+ lung adenocarcinoma. J Thorac Oncol 2012;7:90-7. [Crossref] [PubMed]
- Zhou JX, Yang H, Deng Q, et al. Oncogenic driver mutations in patients with non-Small Cell lung cancer at various clinical stages. Ann Oncol 2013;24:1319-25. [Crossref] [PubMed]
- Kim MH, Shim HS, Kang DR, et al. Clinical and prognostic implications of ALK and ROS1 rearrangements in never-smokers with surgically resected lung adenocarcinoma. Lung Cancer 2014;83:389-95. [Crossref] [PubMed]
- Wang Z, Yang H, Luo S, et al. Anaplastic lymphoma kinase gene rearrangement predicts better prognosis in NSCLC patients: A meta-analysis. Lung Cancer 2017;112:1-9. [Crossref] [PubMed]
- Mak RH, Hermann G, Aerts HJ, et al. Outcomes by EGFR, KRAS, and ALK Genotype After Combined Modality Therapy for Locally Advanced Non–Small Cell Lung Cancer. JCO Precision Oncol 2018. [Crossref]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-Small Cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Massard G, Renaud S, Reeb J, et al. N2-IIIA non-small cell lung cancer: a plea for surgery! J Thorac Dis 2016;8:S849-54. [Crossref] [PubMed]
- Rocco G, Nason K, Brunelli A, et al. Management of stage IIIA (N2) non–small cell lung cancer: A transatlantic perspective. J Thorac Cardiovasc Surg 2016;151:1235-8. [Crossref] [PubMed]
- Samson P, Patel A, Crabtree TD, et al. Multidisciplinary Treatment for Stage IIIA Non-Small Cell Lung Cancer: Does Institution Type Matter? Ann Thorac Surg 2015;100:1773-9. [Crossref] [PubMed]
- Antonia SJ, Özgüroğlu M. Durvalumab in Stage III Non-Small Cell Lung Cancer. N Engl J Med 2018;378:869-70. [PubMed]
- Preoperative chemotherapy for non-Small Cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Song WA, Zhou NK, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. J Thorac Oncol 2010;5:510-6. [Crossref] [PubMed]
- Zhao Y, Wang W, Liang H, et al. The Optimal Treatment for Stage IIIA-N2 Non-Small Cell Lung Cancer: A Network Meta-Analysis. Ann Thorac Surg 2019;107:1866-75. [Crossref] [PubMed]
- Thomas M, Rübe C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-Small Cell lung cancer. Lancet Oncol 2008;9:636-48. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-Small Cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer 2006;94:1099-106. [Crossref] [PubMed]
- Betticher DC, Schmitz S-FH, Tötsch M, et al. Mediastinal Lymph Node Clearance After Docetaxel-Cisplatin Neoadjuvant Chemotherapy Is Prognostic of Survival in Patients With Stage IIIA pN2 Non–Small Cell Lung Cancer: A Multicenter Phase II Trial. J Clin Oncol 2003;21:1752-9. [Crossref] [PubMed]
- Pisters KM, Kris MG, Gralla RJ, et al. Pathologic complete response in advanced non-Small Cell lung cancer following preoperative chemotherapy: implications for the design of future non-Small Cell lung cancer combined modality trials. J Clin Oncol 1993;11:1757-62. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-Small Cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Table of surrogate endpoints that were the basis of drug approval or licensure. Available online: :https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure
- Hellmann MD, Chaft JE, William WN, et al. Pathological response after neoadjuvant chemotherapy in resectable non-Small Cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Weissferdt A, Pataer A, Vaporciyan AA, et al. Agreement on Major Pathological Response in NSCLC Patients Receiving Neoadjuvant Chemotherapy. Clin Lung Cancer 2020;21:341-8. [Crossref] [PubMed]
- Travis WD, Dacic S, Wistuba I, et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 2020;15:709-40. [Crossref] [PubMed]
- Artal Cortés Á, Calera Urquizu L, Hernando Cubero J. Adjuvant chemotherapy in non-small cell lung cancer: state-of-the-art. Transl Lung Cancer Res 2015;4:191-7. [PubMed]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N Engl J Med 2005;353:1659-72. [Crossref] [PubMed]
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 2017;377:122-31. [Crossref] [PubMed]
- Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med 2017;377:1813-23. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs. Three Years of Adjuvant Imatinib for Operable Gastrointestinal Stromal Tumor: A Randomized Trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Wu YL, Zhong W, Wang Q, et al. CTONG1104: Adjuvant gefitinib versus chemotherapy for resected N1-N2 NSCLC with EGFR mutation—Final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial. J Clin Oncol 2020;38:abstr 9005.
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: A Phase II Trial of Adjuvant Erlotinib in Patients With Resected Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer. J Clin Oncol 2019;37:97-104. [Crossref] [PubMed]
- Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-Small Cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med 2018;6:863-73. [Crossref] [PubMed]
- Li N, Ou W, Ye X, et al. Pemetrexed-Carboplatin Adjuvant Chemotherapy With or Without Gefitinib in Resected Stage IIIA-N2 Non-Small Cell Lung Cancer Harbouring EGFR Mutations: A Randomized, Phase II Study. Ann Surg Oncol 2014;21:2091-6. [Crossref] [PubMed]
- Feng S, Wang Y, Cai K, et al. Randomized Adjuvant Chemotherapy of EGFR-Mutated Non-Small Cell Lung Cancer Patients with or without Icotinib Consolidation Therapy. PLoS One 2015;10:e0140794. [Crossref] [PubMed]
- Goss GD, O'Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-Small Cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2015;33:4007-14. [Crossref] [PubMed]
- O'Brien MER, Kelly K, Altorki NK, et al. Final follow-up (f/u) results from RADIANT: A randomized double blind phase 3 trial of adjuvant erlotinib (E) versus placebo (P) following complete tumor resection in patients (pts) with stage IB–IIIA EGFR positive (IHC/FISH) non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr 7540.
- Yuan Y, Huang Q, Gu C, et al. Disease-free survival improved by use of adjuvant EGFR tyrosine kinase inhibitors in resectable non-small cell lung cancer: an updated meta-analysis. J Thorac Dis 2017;9:5314-21. [Crossref] [PubMed]
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Zhong W, Yang X, Yan H, et al. Phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 non-small cell lung cancer based on epidermal growth factor receptor mutation status. J Hematol Oncol 2015;8:54. [Crossref] [PubMed]
- Zhong WZ, Chen KN, Chen C, et al. Erlotinib Versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2. J Clin Oncol 2019;37:2235-45. [Crossref] [PubMed]
- Xiong L, Li R, Sun J, et al. Erlotinib as Neoadjuvant Therapy in Stage IIIA (N2). Oncologist 2019;24:157-e64. [Crossref] [PubMed]
- Zhang Y, Fu F, Hu H, et al. Gefitinib as neoadjuvant therapy for resectable stage II-IIIA non-small cell lung cancer: A phase II study. J Thorac Cardiovasc Surg 2021;161:434-442.e2. [Crossref] [PubMed]
- Tan A, Chua KP, Takano A, et al. P1.17-07 Neoadjuvant Gefitinib in Resectable Early Stage EGFR Mutant Non-Small Cell Lung Cancer (NSCLC): A Window-of-Opportunity Study. J Thorac Oncol 2019;14:S609-10. [Crossref]
- Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791-800. [Crossref] [PubMed]
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-84. [Crossref] [PubMed]
- Rotow J, Urisman A, McCoach C, et al. P1.14-58 A Phase II Study to Evaluate Neoadjuvant Osimertinib for Surgically Resectable, EGFR-Mutant Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:S578. [Crossref]
- Govindan R, Mandrekar SJ, Gerber DE, et al. ALCHEMIST Trials: A Golden Opportunity to Transform Outcomes in Early-Stage Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:5439-44. [Crossref] [PubMed]
- Zhang C, Li SL, Nie Q, et al. Neoadjuvant Crizotinib in Resectable Locally Advanced Non-Small Cell Lung Cancer with ALK Rearrangement. J Thorac Oncol 2019;14:726-31. [Crossref] [PubMed]
- Zenke Y, Yoh K, Sakakibara-Konishi J, et al. P1.18-04 Neoadjuvant Ceritinib for Locally Advanced Non-Small Cell Lung Cancer with ALK Rearrangement: SAKULA Trial. J Thorac Oncol 2019;14:S626-7. [Crossref]
- Provencio M, Nadal E, Insa A, et al. OA13.05 NADIM Study: Updated Clinical Research and Outcomes. J Thorac Oncol 2019;14:S241. [Crossref]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol 2019;30:839-44. [Crossref] [PubMed]
- Calles A, Riess JW, Brahmer JR. Checkpoint Blockade in Lung Cancer With Driver Mutation: Choose the Road Wisely. Am Soc Clin Oncol Educ Book 2020;40:372-84. [Crossref] [PubMed]
- Ren S, Wang C, Shen J, et al. Neoadjuvant immunotherapy with resectable non-small cell lung cancer: recent advances and future challenges. J Thorac Dis 2020;12:1615-20. [Crossref] [PubMed]
- Rusch VW, Chaft JE, Johnson B, et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Initial results from a multicenter study (LCMC3). J Clin Oncol 2018;36:abstr 8541.
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-Small Cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. [Crossref] [PubMed]
- Heymach J, Taube J, Mitsudomi T, et al. P1.18-02 The AEGEAN Phase 3 Trial of Neoadjuvant/Adjuvant Durvalumab in Patients with Resectable Stage II/III NSCLC. J Thorac Oncol 2019;14:S625-6. [Crossref]


