Which patients with extensive stage small-cell lung cancer should and should not receive thoracic radiotherapy?
There is growing awareness of a possible role for consolidation radiotherapy to the chest in patients with extensive stage small-cell lung cancer (ES-SCLC), following completion of chemotherapy (1). In 1999, Jeremic et al. published the results of a study in which patients with ES-SCLC with a complete response after chemotherapy outside the thorax and either a complete or partial response inside the thorax were randomized between prophylactic cranial irradiation (PCI) and additional chemotherapy or PCI, thoracic radiotherapy (TRT) and additional chemotherapy. The authors found that the use of TRT significantly improved survival (2). Since then, a number of non-randomized (3) and retrospective studies (4,5) have also suggested that TRT could be beneficial in patients with ES-SCLC.
The recent results of the CREST study (1) have given rise to some new questions. The study showed that in ES-SCLC patients with any response after chemotherapy, TRT led to a significant improvement in progression-free survival (P<0.001), and a nearly 50% reduction in the risk of intrathoracic progression (P<0.001). The hazard ratio (HR) for overall survival was 0.84 with a 95% confidence interval (CI) just passing through 1.00 (0.69-1.01; P=0.066). Although the survival difference at 1 year was lower than the expected 10% (33% vs. 28%), a significant difference was observed in overall survival at 2 years, where patients receiving both TRT and PCI had a 2-year survival rate of 13%, vs. 3% for the PCI-only arm (1). Statistical purists will, however, still call it a negative study, whereas others will focus on the differences in recurrence patterns, progression-free and overall survival. In this regard, it should be pointed out that overlapping survival curves in the first 9 months after randomization were also observed in the meta-analysis evaluating the role of TRT in limited-stage SCLC (6).
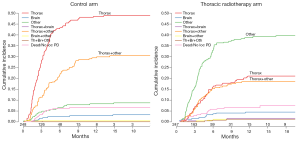
Singer and Yom (7) point to some issues which could have led to a failure to reach a statistically significant difference in overall survival at 12 months. Firstly, progression at extrathoracic sites could have played a role. They also assume that the absence of planned (repeat) brain imaging after chemotherapy would have led to a confounding role for brain metastases. As shown in Figure 1, there was a significant difference in type and rate of progression between the two study arms. In patients receiving PCI only, the majority had early progression in the thorax or combined in thorax with other extracranial and extrathoracic sites. However, in patients who received PCI plus TRT, recurrences occurred later, and were more often observed at extrathoracic and extracranial sites. As brain metastases were observed in only 7% of study patients, it is unlikely that this factor played a major role. Obviously, results could be further improved with a better selection of patients who might benefit from TRT, and by other factors such as increasing the TRT dose and possibly radiotherapy to extrathoracic sites. These issues have been addressed in a phase II randomized RTOG 0937 trial (8). However, as mentioned by Singer and Yom (7), this study was closed prematurely due to futility, and excessive high grade toxicity was observed in the arm receiving PCI plus TRT plus extrathoracic radiotherapy (8). Further analysis of the study might help us to better define the role of higher dose TRT and radiotherapy to other sites of disease.
Van Houtte and colleagues (9) have raised some additional questions. When comparing the results of the CREST-study to other trials, it should be pointed out that survival was measured from randomization after chemotherapy, whereas other studies in the literature measured time from diagnosis or start of first treatment, which is about 4-5 months earlier. We have performed additional analysis to identify patients who may have benefitted most from TRT, and found that TRT led to a significant difference in overall and progression-free survivals in particular patients who had residual intrathoracic disease after chemotherapy (10). In these patients, the difference in overall survival was statistically significant (P=0.03; HR 0.81; 95% CI: 0.66-0.98; stratified). In patients who achieved a complete intrathoracic response, no benefit of TRT was observed. Van Houtte et al. (9) also suggest that the lack of a full reevaluation after chemotherapy might hamper the results. However, almost all patients (97%) had at least a repeat CT of chest and upper abdomen and other sites were only imaged according to existing clinical guidelines. We agree in that a more detailed knowledge on extent and sites of distant metastases could be useful, especially for the design of future studies with higher doses and radiotherapy of extrathoracic sites. Another issue raised is treatment at time of progression. It may indeed be that although the study was properly randomized, more patients in the TRT were fitter and were able to receive second or third line chemotherapy, just as in the EORTC study on PCI (11). There is no easy way to solve that issue in retrospect.
Based on the additional analysis highlighted above (10), we conclude that TRT should be offered to patients with a good or partial response after chemotherapy, but not those without residual disease in the thorax.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Provenance: This is a Guest Article commissioned by the Editorial Board Member Ying Liang [Department of Medical Oncology, Sun Yat-sen University Cancer Center (SYSUCC), Guangzhou, China].
Response to: Singer L, Yom SS. Consolidative radiation therapy for extensive-stage small cell lung cancer. Transl Lung Cancer Res 2015;4:211-4.
Van Houtte P, Moretti L, Roelandts M. Is chest radiation now a classical practice for extensive small cell lung cancer? Transl Lung Cancer Res 2015;4:209-10.
References
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [PubMed]
- Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol 1999;17:2092-9. [PubMed]
- Yee D, Butts C, Reiman A, et al. Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiother Oncol 2012;102:234-8. [PubMed]
- Giuliani ME, Atallah S, Sun A, et al. Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic radiotherapy. Clin Lung Cancer 2011;12:375-9. [PubMed]
- Zhu H, Zhou Z, Wang Y, et al. Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer 2011;117:5423-31. [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [PubMed]
- Singer L, Yom SS. Consolidative radiation therapy for extensive-stage small cell lung cancer. Transl Lung Cancer Res 2015;4:211-4.
- NRG Oncology. RTOG 0937. Available online: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0937
- Van Houtte P, Moretti L, Roelandts M. Is chest radiation now a classical practice for extensive small cell lung cancer? Transl Lung Cancer Res 2015;4:209-10.
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial (Authors’s reply). Lancet 2015;385:1292-3. [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [PubMed]


