18F-FDG-PET/CT in the assessment of pulmonary solitary nodules: comparison of different analysis methods and risk variables in the prediction of malignancy
Introduction
A solitary pulmonary nodule (SPN) is defined radiologically as an intraparenchymal lung lesion of less than 3 cm in diameter, with no associated atelectasis or adenopathy (1,2). The management of SPN is clinically controversial and is mainly dependent on the perceived probability of malignancy (3). The prevalence of lung cancer in patients with SPN varies widely, from 2-13% in screening studies, to 46-82% in positron emission tomography (PET) studies (4,5). For a suspicious malignant SPN, percutaneous transthoracic biopsy, transbronchial needle aspiration biopsy or video-assisted thoracoscopic surgery provides histological information. However, these are invasive procedures, skill-dependent and with variable accuracy to the diagnosis of cancer (6-8).
PET with 18F-fluorodeoxyglucose (FDG) has had an important impact to the diagnosis of benign and malignant nodules. Some reports have suggested that PET can reduce the number of patients with pulmonary nodules who undergo unnecessary surgical biopsy (9). Therefore PET using 18F-FDG is an accurate and noninvasive method for diagnosing SPNs, with an overall sensitivity (Se) of 95% and a specificity (Sp) of 82% (10). However, surgical resection is still needed to differentiate lung cancer from benign lesions in a significant number of cases (6). The combination of computed tomography (CT) and PET in the hybrid imaging, has showed an excellent performance in classifying SPN as benign or malignant, where the Se of CT and the Sp of PET, result in an overall significantly improved accuracy (3,11).
To determine the management and treatment of the patient with a SPN, is necessary to estimate the probability of malignity from clinical and imaging data. Some independent predictors of malignancy include age, current or past smoking history, previous extrathoracic malignancy, nodule diameter, spiculation, and upper lobe location (8,12). Although specific models exist for the calculation of the probability of malignancy of a SPN, they do not have enough accuracy to replace of the clinician’s judgment. On the other hand, adding metabolic parameter derived from PET studies has showed to improve the prediction of malignancy in SPN (11,13), however, it is necessary to increase the evidence that support the use of such metabolic parameters.
FDG uptake on PET has been qualitatively and semiquantitatively evaluated. Visual assessment is usually based upon comparison of FDG lesion uptake with normal mediastinal blood pool (14) and is the simplest among all the analyses, but nodules with similar FDG uptake to the mediastinum are difficult to evaluate visually. In order to have a more objective assessment, a cut-off the maximum standard uptake value (SUVmax) has been used for the establishment of malignancy. However, a great number of factors can affect the SUV, among them, body size, the blood glucose concentration, the time after injection, and the lesion diameter (15). As a result, the SUVmax of a SPN could not reflect its true nature.
In an attempt to improve the diagnostic accuracy of the presurgical evaluation of the SPN, the integration of risk variables into predictive models has been carried out, because, contrary to the clinical judgment, quantitative predictive models might have advantages in accuracy and reproducibility (8,12-14,16). Even though, several CT derived parameters have been included in such predictive model, metabolic variables have been no included.
The purposes of the present study were as follows: (I) to determine an optimum semiquantitative criterion that allows discriminating between malignant and benign nodules and comparing with the visual assessment and (II) to derivate a model to estimate the pretest probability of malignancy of a patient with SPN based on clinical and PET/CT image variables.
Materials and methods
A retrospective evaluation of PET/CT image data, final pathological classification and risk clinical and demographic variables of patients with SPN was performed. The data analysis was carried out after the approval by the Institutional Review Board.
Patients
Between January 2007 and December 2012, patients with a suspicious SPN, underwent a combined whole-body FDG PET/CT imaging and surgical resection of the SPN were included. After surgery, a final histological diagnose was assigned.
Other patient’s characteristics as gender, age and previous or current history of smoking were analyzed.
PET/CT image acquisition and interpretation
Patients fasted for at least 4 h and had blood glucose levels less than 160 mg/dL previous to an intravenous administration of 370 MBq of 18F-FDG.
FDG PET/CT scans were performed approximately 60 min after FDG administration using an integrated PET/CT scanner (Discovery STE 16, GE Healthcare). PET/CT was obtained from the head to proximal thighs. Prior to PET acquisition, helical CT was performed to provide attenuation correction, with acquisition parameters for the CT of 120 kV and modulated 120 mA. No oral or intravenous contrast agents were used. Emission images were acquired in three-dimensional (3D) mode, 3 min per table position. PET images were reconstructed using CT for attenuation correction with ordered-subset expectation maximization iterative reconstruction algorithm. The PET and CT section thickness was 3.8 mm.
Two experienced nuclear medicine physicians reviewed the FDG-PET/CT studies in consensus. In the visual analysis of the PET data, a lesion was defined as negative (no FDG uptake visually detected) or positive (FDG-avid SPN regardless of its intensity).
For semiquantitative analysis, a circular region of interest was placed over the nodule location with the peak activity. The maximum intensity of FDG uptake was defined by body-weight SUVmax measurement using the commercially available software provided by the manufacturer. On the other hand, the nodule diameter (mm) was assessed in axial projection on CT image.
Four metabolic criteria were used to consider a SPN as positive and therefore probably malignant:
- A visually detectable metabolism;
- SUVmax >2.5 regardless of nodule diameter;
- SUVmax ≥1 if diameter ≤1 cm or SUVmax >2.5 if diameter >1 cm;
- Ratio SUVmax/SPN diameter >1.
Final diagnosis
All patients underwent surgical resection of the SPN. A definitive pathologic diagnosis of the SPN, classifying the lesions as benign or malignant, was established.
Statistical analysis
Statistical analysis was performed using SPSS for windows version 19.0 (IBM, Armonk, New York, USA). All the comparisons were two-sided using a P value less than 0.05 to indicate statistical significance.
An independent t-test was used for comparing the age, diameter and SUVmax of the benign and malignant nodules, while that chi-square was used for smoking history, and gender. The diagnostic accuracy was obtained for each of the four different diagnostic approaches. A positive SPN classified by any of the four criteria was considered malignant in the metabolic assessment.
A receiver operating characteristic (ROC) curve analysis was performed to obtain the best cut-off of the SUVmax and SUVmax/diameter (diagnostic approaches III and IV), and the areas under curve (AUC) values of were obtained with a confidence interval (CI) of 95%.
Finally, we developed a model to estimate the probability of malignancy of patients with SPN by using stepwise logistic regression, with the final diagnosis as the dependent variable and the following independent variables: age, gender, smoking history (never vs. ever), nodule size, and SUVmax. Using backward selection, we achieved a final reduced model by eliminating variables that were not statistically significant at a level of 0.05. We used this final model to calculate the estimated probability of malignancy in each patient. We compared the predicted probability of malignancy with the final diagnosis and constructed a ROC curve. To describe the accuracy of the model for identifying malignancy in the patients, we reported the AUC with a CI of 95%.
Results
Fifty-five patients with SPN (45 men and 10 women, with a mean age of 62±11 years) were studied.
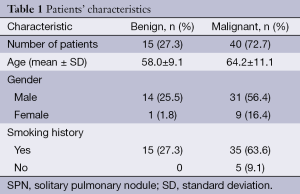
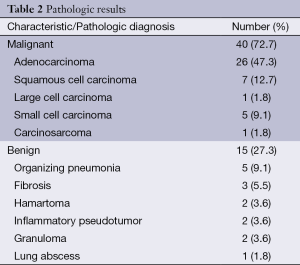
The pathologic analysis classified 40 (72.7%) of SPN as malignant and 15 (27.3%) as benign. From malignant SPN, the most prevalent histologies were: 65% adenocarcinoma, 17.5% epidermoid and 12.5% small cell carcinoma. Among the benign SPN, the most prevalent histologies were: 40% organizing pneumonia and 20% fibrosis. Patient demographics, smoking history and SPN characteristics attending the final pathologic diagnosis of the SPN are shown in Tables 1 and 2.
Full table
Full table
Mean ± standard deviation (SD) values of SPN diameter and SUVmax were 1.93±0.57 cm and 3.93±2.67, respectively.
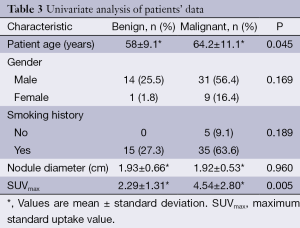
There were statistically significant differences between the SUVmax values and patient age with the final histology of the SPN (malignant or benign). The mean ± SD of the SUVmax for benign nodules was 2.29±1.31 and 4.54±2.80 for malignant nodules (P=0.005). The mean patient age was 58±9 and 64±11 for benign and malignant SPN respectively, (P=0.045). No statistically significant differences were found for the rest of variables (Table 3).
Full table
Se, Sp and diagnostic accuracy for the different diagnostic criteria were (I): 97.5%, 13.1% and 74.5%; (II) 67.5%, 53.3% and 63.3%; (III) 70%, 53.3% and 64.5%; (IV) 85%, 33.3% and 70.9%, respectively.
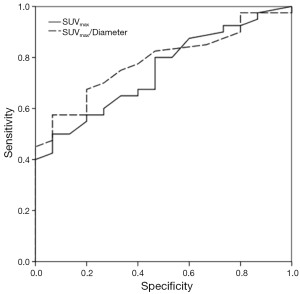
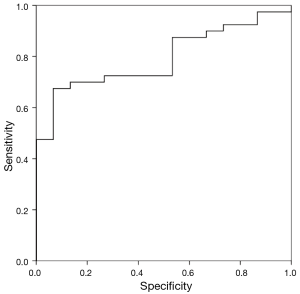
ROC analysis showed an AUC for SUVmax and SUVmax/diameter of 0.75 and 0.79 (P <0.005), respectively.
The cutoff values with the best diagnostic performance were 1.95 (Se: 80%, Sp: 53.3%) and 1.04 (Se: 82.5%, Sp: 53.3%) for SUVmax and SUVmax/diameter, respectively. Figure 1 shows the ROC curves.
By using univariate analysis, we identified that age and SUVmax were associated to malignity (Table 3). However, only SUVmax was an independent predictor in the multivariate analysis, with odd ratio of 1.6 and (95% CI, 1.01-2.417), see Table 4. Although age was not an independent variable, it was included in the predictive model, because its clinical importance, becoming to be an independent predictor in patients older than 60 years.
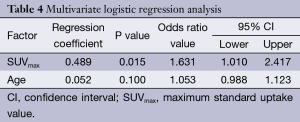
Full table
All other variables were not predictors of malignity, and therefore were not included in the final model. The prediction model is described by the following equations:
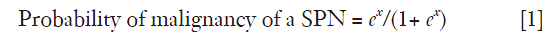
Where x =−3.767+ (4.89× SUVmax) + (0.052× Age), e is the base of the natural logarithm, Age is the age in years and SUVmax is the maximum uptake value on the PET. The accuracy of the model was good with an AUC of the ROC curve of 0.793 (95% CI, 0.676-0.911, P<0.001), with Se and Sp of 87.5% and 46.7% respectively (Figure 2).
Discussion
The diagnosis of SPN remains a major challenge in medical practice. Detecting and diagnosing SPN is critical, as early identification of malignant nodules improves the chance for successful treatment.
With regard to the FDG PET/CT imaging, some interpretation approaches have been assessed. Attending to visual assessment, a broad range of Se and Sp have been reported with values ranging from 69% to 100% and 63% to 85%, respectively (3,11,13,17). We found for the visual assessment (criterion I), a Se of 97.5%, it is in accordance with previously reported results, although the Sp (13.1%) was very limited, which is probably explained by the high prevalence of malignancy in our sample of patients. It is well know that higher the prevalence is, lower the risk of false positive results, and the prevalence will be higher as the inclusion of individuals in the screening program becomes more selective, focusing on higher clinical risk.
Abnormal 18F-FDG uptake is not specific for malignancy; some benign lesions such as bacterial pneumonia, active sarcoidosis, infectious granulomas, acute pyogenic abscesses, cryptogenic fibrosing alveolitis, and so forth have been known to produce false-positive readings on PET (18). In our sample of patients, 27.3% of lesions were finally classified as benign, and from them the most prevalent were organizing pneumonia (40%), fibrosis (20%), and granulomas (13.3%). The median SUVmax for the benign lesions were 2.29±1.31, while that for malignant lesions were 4.54±2.80 (P<0.001).
In an attempt to improve the accuracy of the metabolic assessment some semiquantitative procedures have been developed. For instance, the uptake of the SPN (i.e., the glucose utilization) can be semiquantitatively assessed by the SUVmax and the uptake relative to the background activity in the uninvolved adjacent lung parenchyma and the mediastinum (19).
When we used a semiquantitative method, the Sp increased with a decreasing in sensibility and accuracy. The criterion (II), using a SUV cut-off of 2.5 regardless of the nodule size, had a sensibility, accuracy and Sp of 67.5%, 63.3% and 53.3% respectively. However, these parameters have been reported to be higher. A meta-analysis reported pooled Se of 95% (95% CI, 0.93-0.98) and Sp of 82% (95% CI, 0.77-0.88) to malignant nodules (10).
Partial volume effect and motion during the scan acquisition affects the uptake values measurement, especially for lesions smaller than about three times the spatial resolutions of the equipment, so partial volume and motion corrections factors for standardized PET uptake values may significantly change the differential diagnosis of small pulmonary nodules (20). In order to take into account volume partial effect, we used two different approximations to consider a SPN as malign, (criterion III): a variable threshold of SUVmax depending on the SPN diameter, and (criterion IV): the value obtained by dividing the SUVmax between the diameter of the nodule. This approach is justified, because SUVmax measure is affected by the nodule size, and although it is possible to use a recovery coefficient to have more accurate measurement (21), we use the nodule size, since it is proportional to the recovery coefficient.
The respiratory movement reduces the Se to detect pulmonary lesions; however, the synchronized acquisition of PET with respiratory movement (4D PET) can reduce this inconvenient. When the 4D PET is used to evaluate faint pulmonary lesions there is an increase of SUVmax respect to 3D (22,23). Even when this modality of acquisition was not used in our patient group, we expect to apply it to develop future works.
We aimed to assess the diagnostic accuracy of FDG-PET/CT, as well as to identify predictive factors of malignancy in SPN. With respect to the ROC analysis, the best cut-off for the SUVmax was 1.95 vs. 1.04 for the index SUVmax/diameter. Both values showed a Se of 80% and 82.5% respectively, with the same Sp (53.3%). There was an improvement of the diagnostic parameters, especially for the Sp. Our values of Se and Sp were similar to others published. For instance, Kim et al. (21) found that a SUVmax value of 2.5 had a Se and Sp of 89% and 51%, respectively, for all lesion sizes. Also Grgic et al. (13) obtained a Se and Sp of 96% and 55%, respectively.
Age has been reported to be one important risk factors for SPN malignancy (8,14). In our study, we found a statistically significant association between age and malignancy, as has been described. However, it was not an independent predictor of malignity.
The lesion diameter is also an important risk factor for malignancy. Numerous studies have confirmed this finding, always associating lesion growth with its malignant potential. Nodules of more than 20 mm in diameter have a greater than 50% chance of being diagnosed as malignant (20,24). This is not consistent with the findings of the present study, in which we did not find a significant association between lesion diameter and malignancy. We believe that the small size of the sample might have influenced this result.
Smoking has been found as independent predictor of malignancy (4,8,14). In our population the majority of patients had smoking history. Because of low percentage of non-smokers, our population was biased. It might have influenced the results, since we did not find relation between smoking history with the SPN malignancy. An interesting fact is that even, when the principal histological types related to smoking are squamous cell carcinoma and small cell carcinoma (25), we had low prevalence of these histologic types.
The retrospective nature of the study and the selection criteria could affect our results especially the latter. The fact that all the included patients with PET/CT were undergone surgery implied a high pre-test probability of malignancy that biases the PET/CT Sp. However, that warrantied the final histopathological confirmation of all lesions.
With regard to our results, a significant statistical difference between the SUVmax and patient age with final histology of SPN (benign and malignant) was found. This is in accordance with other studies (13,20). However, we found no statistically significant relation between malignancy and factors previously described as predictors of malignancy, such as smoking status, gender, and nodule diameter.
Predictive models of SPN malignancy is of major interest to clinicians. We derived a model to predict the probability of malignancy by a multivariate regression analysis, and identified the SUVmax as only independent predictors of malignancy of SPN. Our model had a Se and a Sp of 92.5% and 66.7%, respectively. Unlike other models (12,14,20), in which only clinical and morphological variables have been used, our model includes the SUVmax as a metabolic variable. The results obtained in this preliminary study allow us to conclude that the SUVmax is a good predictor of malignancy in a SPN and can be used in the diagnostic setting whenever available.
On the other hand, it will be necessary to develop new predictor models of malignancy based on clinical, morphological and metabolic variables, and test their validity.
The use of invasive diagnostic methods, such as fine-needle puncture, has risks to the patients, such as pneumothorax, bleeding and dissemination of the tumor along the trajectory of the needle (26). On the other hand, surgical lung biopsy has a mortality rate of around 0.6% (27). An accurate, robust and efficient predictive models for SPN malignity, it could provide clinicians with reliable information to avoid the need for an invasive diagnostic methods, allowing to limit the management of a SPN with a safe clinical monitoring.
Our predictive model of the SPN malignancy, unlike to other models, used the metabolic variable SUVmax, showing that it is an independent variable to predict malignancy. The diagnostic performance of this model was higher than visual and semiquantitative methodologies.
Conclusions
The assessment of SPN by semiquantitative methods did not improve the sensibility of visual analysis. The limited specificity was independent of the method used. However, the predictive model combining SUVmax and age was the best diagnostic approach, showing the SUVmax to be an independent variable to predict malignancy of a SPN.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [PubMed]
- Sim YT, Poon FW. Imaging of solitary pulmonary nodule-a clinical review. Quant Imaging Med Surg 2013;3:316-26. [PubMed]
- Chang CY, Tzao C, Lee SC, et al. Incremental value of integrated FDG-PET/CT in evaluating indeterminate solitary pulmonary nodule for malignancy. Mol Imaging Biol 2010;12:204-9. [PubMed]
- Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:108S-130S.
- Tan BB, Flaherty KR, Kazerooni EA, et al. The solitary pulmonary nodule. Chest 2003;123:89S-96S. [PubMed]
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Bogot NR, Shaham D. Semi-invasive and invasive procedures for the diagnosis and staging of lung cancer. II. Bronchoscopic and surgical procedures. Radiol Clin North Am 2000;38:535-44. [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Nomori H, Watanabe K, Ohtsuka T, et al. Visual and semiquantitative analyses for F-18 fluorodeoxyglucose PET scanning in pulmonary nodules 1 cm to 3 cm in size. Ann Thorac Surg 2005;79:984-8; discussion 989. [PubMed]
- Cronin P, Dwamena BA, Kelly AM, et al. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology 2008;246:772-82. [PubMed]
- Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med 2007;48:214-20. [PubMed]
- Li Y, Wang J. A mathematical model for predicting malignancy of solitary pulmonary nodules. World J Surg 2012;36:830-5. [PubMed]
- Grgic A, Yüksel Y, Gröschel A, et al. Risk stratification of solitary pulmonary nodules by means of PET using (18)F-fluorodeoxyglucose and SUV quantification. Eur J Nucl Med Mol Imaging 2010;37:1087-94. [PubMed]
- Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005;128:2490-6. [PubMed]
- Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med 2008;49:1804-8. [PubMed]
- Gould MK, Ananth L, Barnett PG, et al. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131:383-8. [PubMed]
- Hashimoto Y, Tsujikawa T, Kondo C, et al. Accuracy of PET for diagnosis of solid pulmonary lesions with 18F-FDG uptake below the standardized uptake value of 2.5. J Nucl Med 2006;47:426-31. [PubMed]
- Metser U, Even-Sapir E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): accumulated data from four years of experience with PET/CT. Semin Nucl Med 2007;37:206-22. [PubMed]
- Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med 2009;50 Suppl 1:11S-20S. [PubMed]
- Khalaf M, Abdel-Nabi H, Baker J, et al. Relation between nodule size and 18F-FDG-PET SUV for malignant and benign pulmonary nodules. J Hematol Oncol 2008;1:13. [PubMed]
- Kim SC, Machac J, Krynyckyi BR, et al. Fluoro-deoxy-glucose positron emission tomography for evaluation of indeterminate lung nodules: assigning a probability of malignancy may be preferable to binary readings. Ann Nucl Med 2008;22:165-70. [PubMed]
- García Vicente AM, Soriano Castrejón AM, Talavera Rubio MP, et al. (18)F-FDG PET-CT respiratory gating in characterization of pulmonary lesions: approximation towards clinical indications. Ann Nucl Med 2010;24:207-14. [PubMed]
- García Vicente AM, Soriano Castrejón A, Talavera Rubio P, et al. (18)F-FDG PET-CT and respiratory synchronization: effect in the detection and catalogation of pulmonary lesions. Rev Esp Med Nucl 2009;28:181-7. [PubMed]
- Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 2004;231:164-8. [PubMed]
- Sachs S, Fiore JJ. An overview of lung cancer. Respir Care Clin N Am 2003;9:1-25. [PubMed]
- Wiener RS, Wiener DC, Gould MK. Risks of Transthoracic Needle Biopsy: How High? Clin Pulm Med 2013;20:29-35. [PubMed]
- van Rens MT, de la Rivière AB, Elbers HR, et al. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest 2000;117:374-9. [PubMed]


