
Pretreatment neutrophil-to-lymphocyte ratio predicts treatment efficacy and prognosis of cytotoxic anticancer drugs, molecular targeted drugs, and immune checkpoint inhibitors in patients with advanced non-small cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer-related death worldwide (1). However, treatment outcomes of patients with lung cancer have improved since the introduction of immune checkpoint inhibitors (ICIs) in 2015. ICIs have not only improved the prognosis of lung cancer, but of many other cancers, such as malignant melanoma and renal cell carcinoma, and also have revealed a new finding that some patients can have a durable response as if the disease has been cured. Despite this accomplishment, however, responses to ICIs vary among patients (2).
Among ICIs, PD-(L)1 inhibitors which block PD-1 pathway for cancer immunotherapy, have been demonstrated to be effective for advanced non-small cell lung cancer (NSCLC) in large phase III studies (3-5). Nonetheless, only approximately 20% of patients had a long-term response, and 30–40% of patients had no response (3,4,6). While the expression of PD-L1, as a biomarker for PD-(L)1 inhibitors, correlates with prolonged survival, PD-(L)1 inhibitors have also been shown to be effective in PD-L1-negative patients, and therefore PD-L1 is insufficient as a reliable marker for selecting patients (3,4,7). This uncertainty is attributable to changes in PD-L1 expression over time, heterogeneous PD-L1 expression in tumor tissues, and non-standardized assessment approaches for companion diagnostics in immunostaining for PD-L1. Other possible biomarkers of ICI efficacy include tumor mutation burden, which seems promising but has not been clinically validated (8). There is thus an increasing need to identify novel biomarkers suitable for patient selection.
The tumor microenvironment, which is mostly composed of invading inflammatory cells, has recently been shown to be deeply involved in the mechanisms of tumor progression, such as tumor growth and metastasis (9). More specifically, lymphocytes, as an inflammatory cell component, play a role in antitumor immunity and therefore are widely used as a measure of immunocompetence (10,11). Conversely, neutrophils, as another inflammatory cell component augmented in response to inflammation, induce the production of chemokines and cytokines, which enhance tumor growth, invasion, and angiogenesis, and therefore are recognized as a factor connecting inflammation to tumor progression (12,13). The neutrophil-to-lymphocyte ratio (NLR), the ratio of these two components, is a convenient inflammatory parameter based only on blood cell components, in addition to other blood cell-based inflammatory markers such as platelet-to-lymphocyte ratio and monocyte-to-lymphocyte ratio. Among them, the most cumulative evidence is currently available for NLR.
Before the advent of immunotherapy in 2015, NLR was clinically studied as a measure of the systemic inflammatory reaction in critically ill patients with benign diseases and as a prognostic predictor of malignant diseases (14). The utility of NLR as a prognostic predictor in malignant diseases has mainly been reported in relation to surgical therapy or radiotherapy, and few reports have investigated its use for predicting the efficacy or prognosis of systemic treatments with cytotoxic anticancer drugs or molecular targeted drugs (15-18). After the advent of immunotherapy, however, it has increasingly been suggested that NLR might be able to predict the efficacy and prognosis in relation to systemic treatment with ICIs (19,20).
We therefore conducted a comprehensive cross-sectional cohort study to determine if pretreatment NLR could predict the treatment efficacy and prognosis of various systemic treatments, including not only ICIs but also cytotoxic anticancer drugs and molecular targeted drugs, in patients with advanced NSCLC.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-777).
Methods
Patient population
Patients with stage IIIB/IV NSCLC who received first-line systemic treatment at Teikyo University Hospital between January 2008 and March 2019 were included in the study. Lung cancer was staged according to the TNM classification, 7th edition (21). The following information was extracted from electronic medical charts: age, sex, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), hematology (neutrophil count and lymphocyte count), presence or absence of epidermal growth factor receptor (EGFR) mutation, first-line treatment (cytotoxic anticancer drugs or EGFR inhibitors), presence or absence of ICI treatment in the entire treatment course, time to treatment failure (TTF) for each drug, and overall survival (OS). Furthermore, with cytotoxic anticancer drugs, it was investigated whether the regimen was platinum-based doublet or monochemotherapy. With regard to EGFR inhibitors, the generation of EGFR-TKI and the type of EGFR mutation (Ex19del/L858R/minor mutation) were examined. In patients who received ICIs, the best response to previous systemic treatment and PD-L1 expression were evaluated.
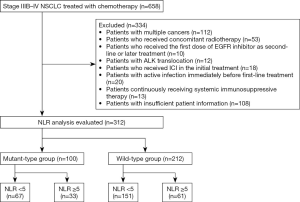
The following patients were excluded from the analysis: those with multiple cancers; those who received concomitant radiotherapy; those who received the first dose of EGFR inhibitor as second-line or later treatment; those with ALK translocation; those who received first-line treatment with ICI; those with active infection immediately before first-line treatment; those continuously receiving systemic immunosuppressive therapy; and those with insufficient patient information.
For patients who received first-line treatment with cytotoxic anticancer drugs or EGFR inhibitors, hematological data collected within 1 week before the first-line treatment were used. For patients who received ICIs, data collected within 1 week before the treatment were used, regardless of the line of therapy.
Patients included in the analysis were classified into patients who received first-line treatment with EGFR inhibitors (mutant-type group: mt group) and patients who received first-line treatment with cytotoxic anticancer drugs (wild-type group: wt group). Using an NLR cut-off value of 5 based on previous reports, each group was further classified into high-NLR (NLR ≥5) and low-NLR subgroups (NLR <5) (19,20).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Teikyo University Ethical Review Board for Medical and Health Research Involving Human Subjects (approved number: 19-146). Due to its retrospective design, informed consent specifically for this study was not required by the Ethical Review Board. For protection of the patient’s personal data, only anonymized data have been used for the analyses of this study.
Statistical methods
The baseline characteristics of patients were compared between the two subgroups using the chi-square test. TTF and OS were estimated using the Kaplan-Meier method and compared between the two subgroups using the log-rank test. TTF was defined as the time from the start date of treatment to the start date of the next treatment or treatment discontinuation for any reason. OS was defined as the time from the start date of treatment to the date of death. TTF and OS were censored at the date of the last follow-up when patients were receiving treatment and were alive, respectively. Multivariate analysis using a Cox proportional-hazards regression model was performed to identify predictors of treatment efficacy and prognosis. In multivariate analysis, PD-L1 status as independent variable was defined as positive if PD-L1 expression was ≥1%. Otherwise, it was defined as negative. A P value <0.05 indicated a significant difference. All statistical analyses were performed using EZR software, version 1.36 (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Results
Patient characteristics
A flow diagram of the study is presented in Figure 1. Of 658 extracted patients, 312 were included in the analysis. There were 100 patients in the mt group and 212 in the wt group.
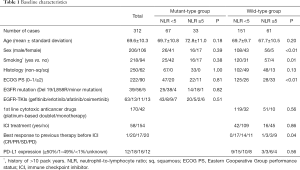
The baseline characteristics of the patients are presented in Table 1. In the mt group, there were no differences in background factors between the high- and low-NLR subgroups, and no patients had squamous cell carcinoma or used ICIs. In contrast, the wt group included significantly more men (P<0.01), smokers (P=0.01), and patients with poor PS (P<0.01) in the high-NLR subgroup compared with the low-NLR subgroup.
Full table
Relationships between NLR and TTF/OS in mt group
In the mt group, the median TTF and median survival time (MST) were 11.5 and 27.4 months, respectively.
The median TTF was significantly lower in the high-NLR subgroup (6.7 months, n=33) compared with the low-NLR subgroup (14.9 months, n=67) (log-rank test, P<0.01) (Figure 2A).
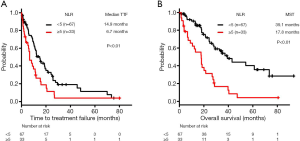
Regarding OS, the MST was significantly lower in the high-NLR subgroup (17.8 months, n=33 compared with the low-NLR subgroup (39.1 months, n=67) (log-rank test, P<0.01) (Figure 2B).
Relationships between NLR and TTF/OS in wt group
In the wt group, the median TTF and MST were 4.4 and 14.5 months, respectively.
The median TTF was significantly lower in the high-NLR subgroup (1.5 months, n=61) compared with the low-NLR subgroup (5.8 months, n=151) (log-rank test, P<0.01) (Figure 3A).
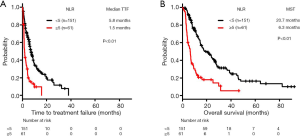
The MST for OS was significantly lower in the high-NLR subgroup (6.3 months, n=61) compared with the low-NLR subgroup (20.7 months, n=151) (log-rank test, P<0.01) (Figure 3B).
Relationships between NLR and TTF/OS in patients treated with ICI
In all ICI-treated patients (n=58), the median TTF and MST were 4.2 and 24.0 months, respectively.
The median TTF was significantly lower in the high-NLR subgroup (1.3 months, n=16) compared with the low-NLR subgroup (6.8 months, n=42) (log-rank test, P<0.01) (Figure 4A).
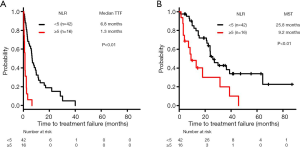
The MST for OS was significantly lower in the high-NLR subgroup (8.8 months, n=16) compared with the low-NLR subgroup (27.1 months, n=42) (log-rank test, P<0.01) (Figure 4B).
Predictive factors for TTF/OS in mt group
In the mt group, multivariate analysis identified ECOG PS [hazard ratio (HR): 2.29, 95% confidence interval (CI): 1.38–3.80; P<0.01], EGFR minor mutation [HR: 3.88, 95% CI: 1.42–10.62; P<0.01], and NLR (HR: 1.89, 95% CI: 1.15–3.09; P=0.01) as significant independent predictors of TTF (Table 2).
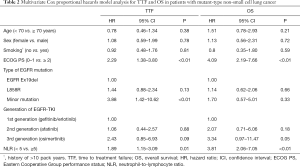
Full table
Similarly, ECOG PS (HR: 4.09, 95% CI: 2.19–7.66; P<0.01) and NLR (HR: 3.81, 95% CI: 2.06–7.05; P<0.01) were identified as significant independent predictors of OS (Table 2).
Predictive factors for TTF/OS in wt group
In the wt group, multivariate analysis identified Histology (HR: 1.43, 95% CI: 1.02–2.01; P=0.03), ECOG PS (HR: 1.89, 95% CI: 1.36–2.64; P<0.01) and NLR (HR: 2.51, 95% CI: 1.77–3.58; P<0.01) as significant independent predictors of TTF (Table 3).

Full table
Similarly, ECOG PS (HR: 1.97, 95% CI: 1.36–2.86; P<0.01), NLR (HR: 2.59, 95% CI: 1.73–3.87; P<0.01), 1st line cytotoxic anticancer drugs (HR: 2.00, 95% CI: 1.25–3.21; P<0.01), and ICI treatment (HR: 2.36, 95% CI: 1.55–3.61; P<0.01) were identified as significant independent predictors of OS (Table 3).
Predictive factors for TTF/OS in patients treated with ICI
In ICI-treated patients, multivariate analysis identified NLR (HR: 5.06, 95% CI: 2.08–12.3; P<0.01) and best response to previous therapy (HR: 3.77, 95% CI: 1.66–8.55; P<0.01) as significant independent predictors of TTF (Table 4).
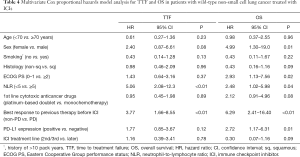
Full table
Similarly, Sex (HR: 4.99, 95% CI: 1.30–19.0; P=0.01), ECOG PS (HR: 2.93, 95% CI: 1.13–7.56; p = 0.02), NLR (HR: 2.48, 95% CI: 1.02–5.98; P=0.04), best response to previous therapy (HR: 6.29, 95% CI: 2.41–16.40; P<0.01), and PD-L1 expression (HR: 2.72, 95% CI: 1.17–6.31; P=0.01) were identified as significant independent predictors of OS (Table 4).
Discussion
This study showed that NLR might be a significant independent predictor of TTF and OS in patients with advanced NSCLC who receive a variety of systemic treatments, including cytotoxic anticancer drugs, molecular targeted drugs, and ICIs, based on a consistent cut-off value.
According to the concept of cancer immunoediting, clinical cancer progression is described as the growth of cancer cells escaping from the immune surveillance system (22). In this situation, PD-L1 on cancer cells may act as an escape molecule for these cells when they are attacked by lymphocytes. ICIs may therefore be expected to exert significant antitumor effects by reactivating lymphocytes in tumors that depend on PD-L1 to escape from lymphocyte attack. However, PD-L1 expression is not always a predictor of treatment efficacy or prognosis for ICI in the clinical setting, and a more reliable predictor is therefore required. On the other hand, neutrophils, which are augmented in response to inflammation, induce the production of reactive oxygen species and chemokines, such as tumor necrosis factor-α, interleukin (IL)-1/-6/-8, interferon α, granulocyte-colony stimulating factors (G-CSFs), and granulocyte-macrophage-colony stimulating factors (GM-CSFs), which enhance tumor growth, invasion, and angiogenesis, and are therefore recognized as a factor connecting inflammation to tumor progression (12,13,23,24). Hence, NLR, the ratio between lymphocytes, which play a central role in antitumor immunity, and neutrophils, which are deeply involved in tumor progression, may affect the efficacy of ICIs.
Indeed, it was first shown in 2015 that NLR before ipilimumab therapy was highly correlated with progression-free survival (PFS) and OS in patients with malignant melanoma (20). More recently, it was reported that pretreatment NLR was significantly correlated with OS in lung cancer patients treated with nivolumab (19,25). Interestingly, a prospective study exploring prognostic predictors for nivolumab in patients with lung cancer showed a significantly better prognosis in patients with pretreatment NLR <5 (n=7) than in patients with NLR ≥5 (n=5), irrespective of high expression of PD-L1 (n=12) [MST: 11.9 months (NLR <5) vs. 3.2 months (NLR ≥5), P=0.0003] (26). These findings indicated that NLR before ICI treatment might be a convenient surrogate marker for the host’s immune status.
In addition to NLR before ICI treatment, the relationship between NLR before molecular targeted therapy and prognosis has been investigated (27-32). It is conventionally assumed that cancer cell growth induced by driver gene alterations such as EGFR mutation and ALK translocation is primarily dependent on the activation of intracellular signaling and is largely unaffected by the cancer microenvironment, which is related to antitumor immunity. Nonetheless, the results of our current study suggested that NLR, which may reflect the cancer microenvironment, predicted the efficacy of EGFR/ALK inhibitors in the mt group. This may be explained by several hypotheses. Molecular targeted drugs tended to normalize neutrophil inflammatory markers such as GM-CSF, G-CSF, and IL-1/-6G in patients with NSCLC (33). In addition, since EGFR is also expressed on regulatory T cells, a subset of T cells, there is a possible mechanism by which EGFR inhibitors may stimulate cytotoxic T cells to attack lung cancer cells by inhibiting the activity of regulatory T cells (34). Due to the aforementioned involvement of molecular targeted drugs in the cancer microenvironment, NLR, as an indicator of activation of immunocompetent cells, may serve as a predictor of treatment efficacy and prognosis in patients with lung cancer induced by driver gene alterations. Further studies are required to clarify the effect of molecular targeted drugs on the cancer microenvironment.
With cytocidal effects primarily targeting DNA and microtubules in cancer cells, cytotoxic anticancer drugs have played a central role in cancer systemic treatment. In recent years, however, the immunotherapeutic properties of cytotoxic anticancer drugs, as well as molecular targeted drugs, are attracting attention. More specifically, immunogenic cell death, by which cancer cells killed by cytotoxic anticancer drugs release an antigen that in turn stimulates dendritic cells to activate cytotoxic T cells, is attracting increasing attention (35). Cytotoxic anticancer drugs have also been shown to exert antitumor effects by inducing cancer cells to produce type I interferon (36). This means that type I interferon-induced release of various antitumor chemokines may stimulate dendritic cells to activate cytotoxic T cells. In addition, cytotoxic anticancer drugs have been reported to directly inactivate immunosuppressive cells such as regulatory T cells and myeloid-derived suppressor cells, which are known to be activated in cancer patients (37). The role of NLR as a predictor of treatment efficacy and prognosis for cytotoxic anticancer drugs, as well as for molecular targeted drugs, remains to be clarified. However, the existence of the aforementioned multiple antitumor immune systems, which are primarily led by lymphocytes, suggests that NLR may indeed serve as a predictor of treatment efficacy and prognosis for cytotoxic anticancer drugs.
To the best of our knowledge, four previous meta-analyses have addressed the cut-off value for NLR as a prognostic predictor in patients with lung cancer, and all four proposed an optimal cut-off value of 5 (38-41). However, differences in the patient population (small cell lung cancer, NSCLC, early stage, and advanced stage) and treatment (surgical therapy, radiotherapy, systemic treatment, and type of systemic treatment) among the articles analyzed in these meta-analyses indicate the need for caution when interpreting the results. On the other hand, a meta-analysis exclusively in patients with advanced NSCLC who received systemic treatment with cytotoxic anticancer drugs, molecular targeted drugs, or ICIs showed the utility of NLR by comprehensively analyzing studies reporting different cut-off values, regardless of the type of systemic treatment (42). With no established cut-off value, the significance of NLR as a predictor of treatment efficacy and prognosis was evaluated in multiple clinical studies conducted to verify the efficacy of nivolumab in advanced NSCLC (19,25,26,43). All studies showed significantly shorter PFS and OS in patients with high NLR based on a cut-off value of 5. We therefore employed an NLR cut-off value of 5 in the current study, and showed that NLR may be a useful predictor of treatment efficacy and prognosis in patients with advanced NSCLC who receive any of the tested systemic treatments, including cytotoxic anticancer drugs, molecular targeted drugs, and ICIs, based on the same cut-off value. We believe that our novel finding of consistent applicability of NLR, which is readily and rapidly available in clinical settings, to a wide variety of systemic treatments is of great significance.
Our study has several limitations. First, since it was a single-center retrospective study, it cannot be denied that various biases may have influenced the results. Indeed, previous reports on NLR had the problem that NLR might be greatly affected by biases resulting from lack of patient entry criteria, including treatment line of systemic treatment (38-41). We therefore made notable efforts to eliminate potential biases affecting the NLR by only selecting patients who received first-line treatment with cytotoxic anticancer drugs or molecular targeted drugs, and excluding patients with active infection immediately before first-line treatment and patients continuously receiving systemic immunosuppressive therapy. Second, since only a limited number of patients received first-line treatment with ICI when our case database was constructed, only patients who received second-line or later treatment with ICI were included in this study. Currently, first-line treatment with ICI alone or in combination with cytotoxic anticancer drugs is widely used as a standard treatment in clinical settings. In the future, combinations of ICIs are likely to be used as a standard treatment. It will therefore be necessary to verify the utility of NLR in relation to these first-line treatments, which were not included in the present study. Third, this study did not investigate the clinical relationship between PD-L1 and NLR, although it is currently standard practice to measure PD-L1 before first-line treatment of advanced NSCLC. This was because only a limited number of patients were analyzed for PD-L1 when our case database was constructed. To enhance the utility of NLR, it will be necessary to determine the relationship between NLR and PD-L1, which is now routinely measured in clinical settings.
Conclusions
The present study showed that NLR might be a useful predictor of treatment efficacy and prognosis in patients with advanced NSCLC who receive a range of systemic treatments, including cytotoxic anticancer drugs, molecular targeted drugs, and ICIs. The novel finding that NLR, which is a readily available and rapid measure in clinical settings, is consistently applicable to a wide variety of systemic treatments is of great significance for the treatment of patients with advanced NSCLC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-777
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-777
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-777). Dr. ST reports personal fees from AstraZeneca outside the submitted work. Dr. NS reports personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Taiho Pharmaceutical, personal fees from Daiichi Sankyo, personal fees from Ono Pharmaceutical, personal fees from Bristol-Myers Squibb, personal fees from MSD Oncology, personal fees from Nihon Medi-Physics, personal fees from Chugai Pharma, personal fees from Lilly Japan, personal fees from Pfizer Japan outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Teikyo University Ethical Review Board for Medical and Health Research Involving Human Subjects (approved number: 19-146). Due to its retrospective design, informed consent specifically for this study was not required by the Ethical Review Board. For protection of the patient’s personal data, only anonymized data have been used for the analyses of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Park YJ, Kuen DS, Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med 2018;50:109. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Huber C, Bobek N, Kuball J, et al. Inhibitors of apoptosis confer resistance to tumour suppression by adoptively transplanted cytotoxic T-lymphocytes in vitro and in vivo. Cell Death Differ 2005;12:317-25. [Crossref] [PubMed]
- Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol 2009;90:222-31. [Crossref] [PubMed]
- Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res 2011;71:2411-6. [Crossref] [PubMed]
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5-14. [PubMed]
- An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010;15:516-22. [Crossref] [PubMed]
- Guthrie GJK, Charles KA, Roxburgh CSD, et al. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- van Soest RJ, Templeton AJ, Vera-Badillo FE, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line systemic treatment: data from two randomized phase III trials. Ann Oncol 2015;26:743-9. [Crossref] [PubMed]
- Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1-7. [Crossref] [PubMed]
- Ferrucci PF, Gandini S, Battaglia A, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer 2015;112:1904-10. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Okamura S, Fujiwara H, Yoneda M, et al. Overexpression of IL-6 by gene transfer stimulates IL-8-mediated invasiveness of KYSE170 esophageal carcinoma cells. Anticancer Res 2013;33:1483-9. [PubMed]
- Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today 1992;13:169-72. [Crossref] [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Fukui T, Okuma Y, Nakahara Y, et al. Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictivebiomarker for advanced non-small-cell lung cancer: a prospective observational study. Clin Lung Cancer 2019;20:208-14.e2. [Crossref] [PubMed]
- Phan TT, Ho TT, Nguyen HT, et al. The prognostic impact of neutrophil to lymphocyte ratio in advanced non-small cell lung cancer patients treated with EGFR TKI. Int J Gen Med 2018;11:423-30. [Crossref] [PubMed]
- Aguiar-Bujanda D, Dueñas-Comino A, Saura-Grau S, et al. Neutrophil to lymphocyte ratio as a prognostic factor in European patients with epidermal growth factor receptor-mutant non-small cell lung cancer treated with tyrosine kinase inhibitors. Oncol Res Treat 2018;41:755-61. [Crossref] [PubMed]
- Zhang Y, Feng YC, Zhu HG, et al. The peripheral blood neutrophil-to-lymphocyte ratio is a prognostic predictor for survival of EGFR-mutant nonsmall cell lung cancer patients treated with EGFR-TKIs. Medicine (Baltimore) 2018;97:e11648. [Crossref] [PubMed]
- Minami S, Ogata Y, Ihara S, et al. Neutrophil-to-lymphocyte ratio predicts overall survival of advanced non-small cell lung cancer harboring mutant epidermal growth factor receptor. World J Oncol 2017;8:180-7. [Crossref] [PubMed]
- Meriggi F, Codignola C, Beretta GD, et al. Significance of neutrophil-to-lymphocyte ratio in Western advanced EGFR-mutated non-small cell lung cancer receiving a targeted therapy. Tumori 2017;103:443-8. [Crossref] [PubMed]
- Chen YM, Lai CH, Rau KM, et al. Impact of clinical parameters and systemic inflammatory status on epidermal growth factor receptor-mutant non-small cell lung cancer patients readministration with epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer 2016;16:868. [Crossref] [PubMed]
- Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res 2015;2015:983698. [Crossref] [PubMed]
- Zaiss DM, van Loosdregt J, Gorlani A, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013;38:275-84. [Crossref] [PubMed]
- Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51-72. [Crossref] [PubMed]
- Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of systemic treatment. Nat Med 2014;20:1301-9. [Crossref] [PubMed]
- Galluzzi L, Buqué A, Kepp O, et al. Immunological effects of conventional systemic treatment and targeted anticancer agents. Cancer Cell 2015;28:690-714. [Crossref] [PubMed]
- Zhao QT, Yang Y, Xu S, et al. Prognostic role of neutrophil to lymphocyte ratio in lung cancers: a meta-analysis including 7,054 patients. Onco Targets Ther 2015;8:2731-8. [PubMed]
- Yang HB, Xing M, Ma LN, et al. Prognostic significance of neutrophil-lymphocyteratio/platelet-lymphocyteratioin lung cancers: a meta-analysis. Oncotarget 2016;7:76769-78. [Crossref] [PubMed]
- Gu XB, Tian T, Tian XJ, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep 2015;5:12493. [Crossref] [PubMed]
- Peng B, Wang YH, Liu YM, et al. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med 2015;8:3098-106. [PubMed]
- Wang Z, Zhan P, Lv Y, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res 2019;8:214-26. [Crossref] [PubMed]
- Cao D, Xu H, Xu X, et al. A reliable and feasible way to predict the benefits of Nivolumab in patients with non-small cell lung cancer: a pooled analysis of 14 retrospective studies. Oncoimmunology 2018;7:e1507262. [Crossref] [PubMed]


