Prognostic factors in potentially resectable stage III non-small cell lung cancer receiving neoadjuvant treatment—a narrative review
Introduction
Lung cancer is the primary cause of cancer-related death in western countries. In 2020, it is expected to account for 228,820 new cases and 135,720 deaths in the USA. It is the second most common solid tumor type in both genders, after prostate cancer in men and breast cancer in women (1). In Spain occur about 29,503 new cases per year and has been responsible for 22,121 deaths in 2017 (2).
Approximately 85% of lung cancer cases are non-small cell lung cancer (NSCLC). Of patients with NSCLC, at diagnosis, 20% present with stage I or II, whereas 30% present with stage III, locally advanced disease, and 50% of patients with stage IV disease.
Patients with stage I NSCLC have a 5-year survival of approximately 68–92%, stage II to III NSCLC patients have a 5-year survival of approximately 25% to 60% (3).
Current standard of care in early and locally advanced NSCLC
In localized stages, stage I and II, surgical resection remains the most important treatment; however, despite the potential curative surgery, approximately 50% of stage IB and 60–75% of stage I–II NSCLC patients will relapse and eventually died from their tumor (4,5).
Many adjuvant studies have been performed, the overall evidence from these studies suggest that adjuvant platinum doublet chemotherapy is beneficial especially in patients with good performance status (PS). The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis included five clinical trials evaluating adjuvant cisplatin-based chemotherapy. The LACE meta-analysis included 4,584 patients and had a median follow-up of 5.2 years. The results demonstrated a 5.4% absolute survival benefit at 5 years [HR: 0.89 (95% CI: 0.82–0.96, P=0.005)]. Survival benefits were seen in stage II and IIIA (6). So as the guides recommend (7), surgery should be offered to all patients with stage I and II NSCLC as the preferred treatment, adjuvant chemotherapy should be offered to patients with resected stage II and III NSCLC and can be considered in patients with resected stage IB disease and a primary tumor >4 cm (8). Several factors are also important when making decisions regarding adjuvant therapy: stage, age, chemotherapy regimens, and timing after surgery.
Neoadjuvant chemotherapy has not been evaluated as extensively as postoperative treatment. However, several meta-analysis of trials evaluating neoadjuvant chemotherapy were conducted. These meta-analyses showed that neoadjuvant chemotherapy improved survival with an absolute benefit of 5–6% at 5 years, which was very similar to the benefit seen with adjuvant chemotherapy (9,10).
The locally advanced disease is when the tumor exceeds the lung structures, but without clinical evidence of distant spread. Stage III NSCLC is a heterogeneous disease. In the 8th edition of the TNM classification proposed by the International Association for the Study of Lung Cancer (IASLC), accepted by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC), stage III NSCLC includes patients who, in the absence of metastatic disease (M0), present N2 or N3 disease, a tumor with T4 characteristics or one classified as T3N1 (11). These patients could have tumors resectable, potentially resectable and unresectable. The treatment is based on a combination of surgery, chemotherapy and radiotherapy.
A few years ago, radiation therapy was considered standard therapy for patients with stage IIIA and IIIB but presented poor survival with poor local control and early development of distant disease.
Patients with stage IIIA disease with clinically evident N2 nodal spread have an overall 5-year survival rate of only 10–15%, although this fall to 2–5% in those with bulky mediastinal N2 involvement.
The surgical management of stage IIIA NSCLC remains highly controversial and most patients with stage IIIB disease (in the 7th edition of the TNM) are generally considered inoperable.
As a matter of fact, it is reported that at least 80% of patients treated with local therapies alone will have micrometastases and will relapse. The goal of treatment in stage III NSCLC is to increase both locoregional and systemic control of the disease.
Neoadjuvant treatment has theoretical advantages: in vivo assessment of response to chemotherapy and this helps to identify patients who will potentially benefit from chemotherapy; early treatment of micrometastatic disease; downstaging with improved resectability and offers the possibility for the identification of surrogate clinical and biological markers that may correlate with response to therapy and a potential long-term outcome. However, neoadjuvant therapy has potential disadvantages: delay in local therapy, like surgery, due to toxicity and pre-operative complications and risk progression of the disease in chemoresistant patients.
Several phase II and III clinical trials comparing induction chemotherapy or surgery directly have been published. It is difficult to compare some trials with others because they have different inclusion criteria, mix patients with different prognosis, what it means resectable or marginally resectable disease and different induction and post-induction treatments (ITs). Despite this, we can conclude that platinum doublet chemotherapy provides an increase in survival (12-17).
In patients with stage IIIA NSCLC, the neoadjuvant therapy outside the clinical trial show a median survival of 22 months and a 3-year survival rate of 34% (18). The identification of those patients who benefit from surgery after induction chemotherapy is controversial. Results such as those of the Southwest Oncology Group (19) in patients with complete pathological response with a median survival of 30 vs. 10 months in those with residual tumor suggest to avoid surgery in those patients with persistence mediastinal involvement after induction.
New agents with proven activity in metastatic disease have been incorporated into ITs such as gemcitabine, paclitaxel (20), vinorelbine and docetaxel (21), with responses ranging from 44% to 80% and complete resection rates ranging from 67% to 79%. And they also have radiosensitizing capacity.
Despite the available treatments, the survival of patients with completely resected NSCLC remains poor and this is the reason why it is necessary to evaluate new strategies of management of these patients.
We present the following article in accordance with the narrative review reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-515).
Methods
We searched PubMed employing the following search words alone or in combination: prognostic factors, NSCLC, stage III, resectable, unresectable, immunotherapy, immune checkpoint inhibitors (ICIs), neoadjuvant and adjuvant treatment.
Discussion
Immune checkpoint inhibition in early-stage NSCLC
Immunotherapy with ICIs has changed the treatment and prognosis in advanced NSCLC. In several large trials, the benefit of the immunotherapy has been demonstrated in patients with advanced NSCLC who had failed a platinum-based doublet. Single-agent ICI has gradually moved from the second- to the first-line setting in patients with advanced NSCLC with a PD-L1 expression ≥50%. Recently, in the first-line setting in patients with advanced NSCLC, the combination of immunotherapy with chemotherapy has been shown to improve survival regardless of the expression of PD-L1 without adding significant toxicity to standard first-line chemotherapy. For patients with unresectable stage III NSCLC, durvalumab after concurrent chemoradiotherapy has already brought a major improvement in 2-year progression-free survival (PFS) and overall survival (OS) rates (22).
In the curative setting, ICIs are being studied in both early and locally advanced stages.
Prognostic factors after neoadjuvant therapy
Typically, we use computed tomography (CT) to evaluate the response to treatment, also to neoadjuvant treatment, but there have been disagreements between the histopathological response rate and the response measured by RECIST which varies between 35% and 41% (23,24).
In different studies, the prognostic of patients with locally advanced NSCLC after neoadjuvant therapy has been based on the change in maximal standard uptake value (SUVmax) on fluorodeoxyglucose positron emission tomography (FDG-PET) scan, tumor size regression, lymph node (LN) status and clinical stage (25-28). These factors are usually evaluated preoperatively, however, sometimes there are significant differences between clinical and pathological stage.
The interpretation of tumor size, inflammatory, stromal or fibrotic components may confuse which would not allow an accurate measurement of the histopathological response after neoadjuvant treatment.
The evaluation of the response to treatment is complicated with the new treatment options such as: the target treatment and immunotherapy. Because of the distinctive biologic mechanism of immune-checkpoint blockade, unconventional tumor response patterns at imaging have been noted in patients treated with ICIs, which antitumor effect does not cause tumor size decrease and where inflammatory effects can influence its size in the images (29). Because of this, the evaluation of the response requires a functional and molecular evaluation. FDG-PET can be used to identify viable tumor but there are also confounding factors such as the avidity for FDG of infiltrated macrophages/monocytes or as the immunological node flare phenomenon which can impress progression by CT and PET but they are only non-caseous granulomas. The observations of Poettgen and collaborators suggest that changes postinduction in SUVmax on FDG-PET scan should be interpreted with caution in larger residual tumor volumes, since high SUVmax levels may be due to macrophage infiltration and not due to viable tumor tissue (30).
The prediction of the histopathological response in patients with NSCLC after neoadjuvant treatment can be more precise using the CT and the FDG-PET together. The accuracy for the prediction of pathologic response was 70% in radiologic responders, 52% to 75% in metabolic responders, and 73% to 82% in radiologic-metabolic responders (31).
After neoadjuvant therapy, complete surgical resection (32,33), tumor downstaging and pathologic complete response are predictors of long-term survival.
Pathologic complete response after induction chemotherapy generally ranges from 0% to 9.5%. Other authors point to higher complete response rates such as Martini (34) with 16.7% or Kumar (35) with 15%.
From the literature review, many prognostic factors have been identified for patients who have been treated with neoadjuvant therapy based on pathology findings (36,37). These factors are: metastatic LN ratio (38), number of residual metastatic LNs (39), smaller area of residual tumor (40) and negative pleural invasion, percentage of viable residual tumor cells (41), and low total macrophage number in the tumor (42).
Andre et al. (43) analyzed a cohort of 702 patients with resected stage IIIA–N2 NSCLC and identified four negative prognostic factors: preoperative clinical N2 status, involvement of multiple LN levels, pathological T3 to T4 stage, and no preoperative chemotherapy. Choi et al. (44) reviewed cases of pathologic proven N2 disease and observed that complete resection rate was 83% and an overall 5-year was 23.3% and 5-year recurrence-free survival was 19.6%. Among 19 clinicopathological prognostic factors, incomplete resection and non-downstaging after neoadjuvant therapy were unfavorable prognostic factors in the univariate analysis. Clinical N2 status, multiple N2 nodes, and cell type of adenocarcinoma (ADC) showed poor prognosis but were not statistically significant. Postoperative chemotherapy showed good prognosis but also did not reach statistical significance. Multivariate analysis showed that significant favorable prognostic factors were complete resection and adjuvant chemotherapy. Experience of Memorial Sloan-Kettering Cancer Center confirms survival is significantly influenced by patient age, the median survival in patients with complete resection was 27.8 vs. 11.4 months in patients with incomplete resection, 3-year survival for pathologic stage N0/N1 was 43.3% and 25.5% for N2 patients (45).
Hsieh et al. (46). designed a study whose aim was to analyze the relationship between clinicopathologic factors and survival from the pathologic point of view and to try to identify survival prognostic factors. They found that the total metastatic LN ratio and tumor size were predictive factors for disease-free survival, tumor size greater than 5 cm and total metastatic LN ratio greater than 0.065 could predict the disease-free survival of patients with advanced NSCLC after multimodality therapies followed by surgical resection.
Marulli et al. (47) evaluated the outcomes and prognostic factors in patients with NSCLC and LN involvement who received IT followed by surgery. A total of 86 patients with stage IIIA NSCLC (n=80) or stage IIIB NSCLC (n=6), with pathologically proven LN-positive, underwent platinum-based neoadjuvant chemotherapy followed by surgery between 2000 and 2009. The median OS was 23 months (5-year survival of 33%). Univariate analysis showed that clinical stage (P=0.02), histology (P=0.01), response to IT (P=0.02) and type of intervention (P=0.047) have predictive roles in survival. Better survival was observed without statistically significant differences for pN0 vs. pN+ (P=0.22), downstaged tumors (P=0.08) and left side (P=0.06). Multivariate analysis showed that the only independent predictors of survival were clinical response to neoadjuvant therapy (P=0.01) and age (P=0.03). In conclusion, neoadjuvant chemotherapy in stage III NSCLC seems to be justified by low morbidity and/or mortality and good survival rates. Patients with response to IT showed more long-term benefit.
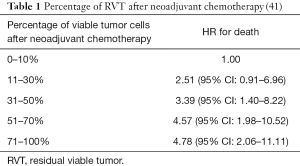
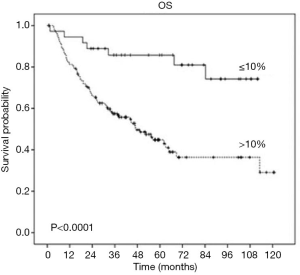
Multiples studies demonstrated that patients with lung cancer who received neoadjuvant treatment and showed a major pathologic response (MPR) (48) defined as 10% or less viable tumor have a significantly improved survival and this is the reason why MPR has been recognized as a predictor of survival and a potential surrogate endpoint in clinical trials. Table 1 (41,48) and Figure 1 (41) shows the robust improvement in survival in patients with 0–10% residual viable tumor (RVT) compared with patients with RVT greater than 10%. Complete pathologic response (CPR) is defined as no viable tumor.
However, few studies have described how the resected surgical specimen of a patient who was treated with neoadjuvant chemotherapy should be evaluated macroscopically and microscopically. Methods of evaluation are described (41) and detailed in Figure 2 (48).
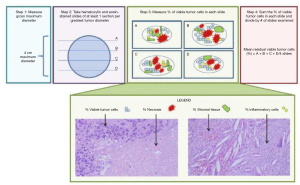
From numerous histopathological criteria reviewed, three major characteristics were determined: necrosis, stromal fibrosis and viable tumor.
It has been reported that these histological changes associated with treatment could also be found in resected lung cancer pieces without neoadjuvant treatment, which is why it is necessary for the pathologist to know the history of the treatment. The histological changes associated with neoadjuvant therapy usually occur on the periphery of the tumor, while in the tumor with spontaneous regression these changes are seen more in the center (49).
Also it has been found association between OS and the nodal response to neoadjuvant treatment (50-53).
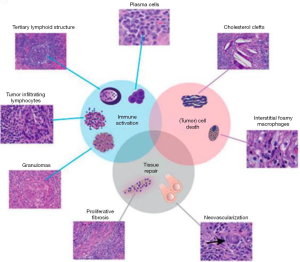
We might think that due to the different mechanisms of action between conventional chemotherapy and immunotherapy, the changes in the pathological response to ICIs could be different from those reported for chemotherapy (Figures 3,4) (49,54,55).
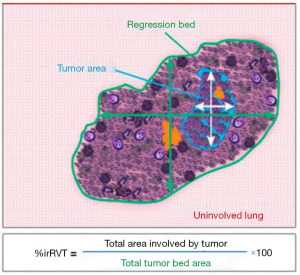
There is also a study that investigate whether the optimal cutoff percentage of RVT for predicting survival differs between lung ADC and squamous cell carcinoma (SCC). Patients with SCC had a better response to neoadjuvant chemotherapy 40% vs. 60% ADC, P=0.027. MPR was observed in 26% of SCC cases vs. 12% of ADC cases (P=0.004). On multivariable analysis, the optimal cutoff percentage of viable tumor for predicting survival differs between ADC and SCC, viable tumor 10% or less was an independent factor for better lung cancer-specific cumulative incidence of death (P=0.035) in patients with SCC; in patients with ADC, viable tumor 65% or less was a factor better lung cancer-specific cumulative incidence of death (P=0.033) and OS (P=0.050) (56).
Summary
In this review article we tried to describe the different prognostic factors in patients with potentially resectable locally advanced NSCLC receiving neoadjuvant treatment. Neoadjuvant setting is considered an opportunity for studying drug mechanism of action and for finding a surrogate for survival, such as pathologic response to neoadjuvant chemotherapy, that could have the potential to improve the efficiency of trials.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research for the series “Multimodal management of locally advanced N2 non-small cell lung cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-515
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-515). The series “Multimodal management of locally advanced N2 non-small cell lung cancer” was commissioned by the editorial office without any funding or sponsorship. MP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Lung Cancer Research from Sep 2019 to Sep 2021. VC reports personal fees: Roche, BMS, MSD, AstraZeneca, Boehringer. MP reports honoraria as speaker and consultant on advisory boards from: Roche, BMS, MSD, Takeda and Lilly. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- REDECAN. Red Española de Registros de Cáncer. Available online: https://redecan.org/redecan.org/es/index.html
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-21. [Crossref] [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatmet Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Burdett SS, Stewart LA, Rydzewska L. Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. Cochrane Database Syst Rev 2007;3:CD006157. [Crossref] [PubMed]
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Rami-Porta R, Call S, Dooms C, et al. Lung cancer staging: a concise update. Eur Respir J 2018;51:1800190. [Crossref] [PubMed]
- Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [Crossref] [PubMed]
- Rosell R, Gomez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer 1999;26:7-14. [Crossref] [PubMed]
- Roth JA, Fosella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-snall-cell luna cancer. Lung Cancer 1998;21:1-6. [Crossref] [PubMed]
- Pass HI, Pogrebniak HW, Steimberg SM, et al. Randomized trial comparing neoadjuvant therapy for lung cancer: interim analysis. Ann Thorac Surg 1992;53:992-8. [Crossref] [PubMed]
- Depierre A, Milleron M, Moro-Sililot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIA non-small lung cancer. J Clin Oncol 2002;20:247-53. [PubMed]
- Martin J, Ginsberg RJ, Ennapadam S, et al. Long-term results of combined-modality therapy in resectable non-small cell lung cancer. J Clin Oncol 2002;20:1989-995. [Crossref] [PubMed]
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery in stages IIIA (N2) and IIIB non-small cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995;13:1880-92. [Crossref] [PubMed]
- O’Brien MER, Splinter T, Smit EF, et al. Carboplatin and paclitaxel (Taxol) as an induction regimen for patients with biopsy-proven stage IIIA N2 non-small cell lung cancer: an EORTC phase II study (EORTC 08958). Eur J Cancer 2003;39:1416-22. [Crossref] [PubMed]
- Zarogoulidis K, Kontakiotis T, Hatziapostolou P, et al. A Phase II study of docetaxel and carboplatin in the treatment of non-small cell lung cancer. Lung Cancer 2001;32:281-7. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- William WN Jr, Pataer A, Kalhor N, et al. Computed tomography RECIST assessmente of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2013;8:222-8. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol 2003;21:2651-7. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Winokur TS, et al. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg 2004;78:1903-9. [Crossref] [PubMed]
- Eschmann SM, Friedel G, Paulsen F, et al. Is standardised (18)F-FDG uptake value an outcome predictor in patients with stage III non-small cell lung cancer? Eur J Nucl Med Mol Imaging 2006;33:263-9. [Crossref] [PubMed]
- Tieu BH, Sanborn RE, Thomas CR. Neoadjuvant therapy for resectable non-small cell lung cancer with mediastinal lymph node involvement. Thorac Surg Clin 2008;18:403-15. [Crossref] [PubMed]
- Nishino M, Hatabu H, Hodi FS. Imaging of cancer immunotherapy: current approaches and future directions. Radiology 2019;290:9-22. [Crossref] [PubMed]
- Poettgen C, Theegarten D, Eberhardt W, et al. Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology 2007;73:316-23. [Crossref] [PubMed]
- Lee HY, Lee HJ, Kim YT, et al. Value of combined interpretation of computed tomography response and positron emission tomography response for prediction of prognosis after neoadjuvant chemotherapy in non-small cell lung cancer. J Thorac Oncol 2010;5:497-503. [Crossref] [PubMed]
- Sugarbaker DJ, Herndon J, Kohman LJ, et al. Results of cancer and leukemia group B protocol 8935. A multiinstitutional phase II trimodality trial for stage IIIA (N2) non-small-cell lung cancer. Cancer and Leukemia Group B Thoracic Surgery Group. J Thorac Cardiovasc Surg 1995;109:473-83; discussion 483-5. [Crossref] [PubMed]
- Kirn DH, Lynch TJ, Mentzer SJ, et al. Multimodality therapy of patients with satge IIIA, N2 non-small-cell lung cancer: impact of preoperative chemotherapy on resectability and downstaging. J Thorac Cardiovasc Surg 1993;106:696-702. [Crossref] [PubMed]
- Martini N, Kris MG, Flehinger BJ, et al. Preoperative chemotherapy for stage IIIa (N2) lung cancer: the Sloan-Kettering experience with 136 patients. Ann Thorac Surg 1993;55:1365-73. [Crossref] [PubMed]
- Kumar P, Herndon J, Elias D, et al. Comparison of pre-operative thoracic radiation therapy (TRT) to pre-operative chemotherapy (CT) in surgically staged IIIA (N2) non-small cell lung cancer (NSCLC): initial results of Cancer and Leucemia Group B (CALGB) phase III protocol 9134. Int J Radiat Oncol Biol Phys 1997;39:195. [Crossref]
- Martini N, Burtt ME, Bains MS, et al. Survival after resection of stage II non-small cell lung cancer. Ann Thorac Surg 1992;54:460-5; discussion 466. [Crossref] [PubMed]
- Sawyer TE, Bonner JA, Gould PM, et al. Factors predicting patterns of recurrence after resection of N1 non-small cell lung carcinoma. Ann Thorac Surg 1999;68:1171-6. [Crossref] [PubMed]
- Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg 2012;93:1614-9; discussion 1619-20. [Crossref] [PubMed]
- Kim SH, Cho BC, Choi HJ, et al. The number of residual metastatic lymph nodes following neoadjuvant chemotherapy predicts survival in patients with stage III NSCLC. Lung Cancer 2008;60:393-400. [Crossref] [PubMed]
- Yamane Y, Ishii G, Goto K, et al. A novel histopathological evaluation method predicting he outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor. J Thorac Oncol 2010;5:49-55. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Feng PH, Yu CT, Wu CY, et al. Tumor-associated macrophages in stage IIIA pN2 non-small cell lung cancer after neoadjuvant chemotherapy and surgery. Am J Transl Res 2014;6:593-603. [PubMed]
- Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 2000;18:2981-9. [Crossref] [PubMed]
- Choi YS, Shim YM, Kim J, Kim K. Recurrence-free survival and prognostic factors in resected pN2 non-small cell lung cancer. Eur J Cardiothorac Surg 2002;22:695-700. [Crossref] [PubMed]
- Martini N. Mediastinal lymph node dissection for lung cancer. The Memorial experience. Chest Surg Clin N Am 1995;5:189-203. [PubMed]
- Hsieh CP, Hsieh MJ, Wu CF, et al. Prognostic factors in non-small cell lung cancer patients who received neoadjuvant therapy and curative resection. J Thorac Dis 2016;8:1477-86. [Crossref] [PubMed]
- Marulli G, Verderi E, Zuin A, et al. Outcomes and prognostic factors of non-small-cell lung cancer with lymph node involvement treated with induction treatment and surgical resection. Interact Cardiovasc Thorac Surg 2014;19:256-62. [Crossref] [PubMed]
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surroget endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Junker K, Thomas M, Schulmann K, et al. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol 1997;123:469-77. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752-9. [Crossref] [PubMed]
- Jaklitsch MT, Herndon JE 2nd, DeCamp MM Jr, et al. Nodal downstaging predicts survival following induction chemotherapy for stage IIIA (N2) non-small cell lung cancer in CALGB protocol #8935. J Surg Oncol 2006;94:599-606. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer 2006;94:1099-106. [Crossref] [PubMed]
- Katakami N, Tada H, Mitsudomi T, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012;118:6126-35. [Crossref] [PubMed]
- Liu-Jarin X, Stoopler MB, Raftopoulos H, et al. Histologic assessment of non-small cell lung carcinoma after neoadjuvant therapy. Mod Pathol 2003;16:1102-8. [Crossref] [PubMed]
- Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018;29:1853-60. [Crossref] [PubMed]
- Qu Y, Emoto K, Eguchi T, et al. Pathologic assessment after neoadjuvant chemotherapy for NSCLC: importance and implications of distinguishing adenocarcinoma from squamous cell carcinoma. J Thorac Oncol 2019;14:482-93. [Crossref] [PubMed]