
Can autoantibody tests enhance lung cancer screening?—an evaluation of EarlyCDT®-Lung in context of the German Lung Cancer Screening Intervention Trial (LUSI)
Introduction
While low dose computed tomography (LDCT) screening has now been broadly documented to have the potential to reduce lung cancer (LC) mortality (1-5), it comes with risks of radiation-induced cancer, false-positive test results, unnecessary follow-up testing and increased financial costs, as well as overdiagnosis (6,7). False-positive test results are a major concern, especially as these can lead to further, more invasive diagnostic verification procedures (surgery, biopsy). In view of improving the specificity of non-invasive lung cancer detection and diagnostic triage, there is extensive ongoing research on the complementary use of biomarkers measured in blood, exhaled breath condensates, or other biological materials (8-14). In addition, biomarkers are also being considered as a possibly more economic tool for population (pre-) screening, e.g., to selectively identify individuals who likely harbor a tumor that may be detectable during follow-up examinations by LDCT (15).
A promising class of detection markers are tumor-associated autoantibodies (TAAbs). These are produced as an immune (B-cell) response to aberrantly expressed, mutated or post-translation zally modified proteins, or other tumor antigens. Comparative immuno-proteomic scans of blood sera from LC patients and cancer-free control subjects have identified multiple TAAbs showing highly increased titers in cancer patients with various forms of solid tumors, including lung cancer. In fact, a number of individual TAAbs and multi-TAAb panels have been evaluated for its ability to discriminate lung cancer patients from cancer-free individuals (16-20). Panels have outperformed individual TAAbs in all studies, which has been attributed to the heterogeneity of lung cancer tumors (17). However, while most of the panels show good specificity, their sensitivity was generally only modest (17,19,20). One well-established panel is EarlyCDT®-Lung (Oncimmune Ltd, Nottingham, United Kingdom) which, in its most recent version, is composed of 7 different antibody assays (CAGE, GBU4-5, HuD, MAGEA4, NY-ESO-1, p53 and SOX2). Most of these are not specific for lung cancer but arise also in other cancers such as breast, colorectal, gastric, prostate, liver and testis, as well as in autoimmune diseases (16-18). This panel has shown useful diagnostic discrimination and has been proposed as a “rule-in” test, with about 90% specificity at 40% sensitivity in clinical studies comparing lung cancer cases and cancer-free control subjects (14,21-23).
It is generally hypothesized that TAAbs are produced at very early stages of tumor development, which would make them suitable candidate markers for early detection (24). So far, however, it has not been studied whether blood TAAb concentrations are elevated in patients with small malignant nodules (<10 mm in diameter) as detected by LDCT screening (25), and whether antibody tests such as EarlyCDT®-Lung can detect tumors in an equally early stage as with LDCT-based screening. To address these questions, we performed EarlyCDT®-Lung tests in blood samples collected according to the protocol of the German Lung Cancer Screening Intervention Trial (LUSI) (4,26) and examined the sensitivity and specificity of this test panel for the detection of pulmonary tumors.
We present the following article in accordance with the Standards for Reporting of Diagnostic Accuracy Studies (STARD) reporting checklist (27) (available at http://dx.doi.org/10.21037/tlcr-20-727).
Methods
LUSI trial design
The German Lung Cancer Screening Intervention (LUSI) (4,26) is a screening trial among men and women from the Heidelberg region in Germany. Individuals eligible for the LUSI trial were those with a history of long-term smoking (smoking at least 15 cigarettes per day for at least 25 years, or smoking at least 10 cigarettes per day for 30 years, including ex-smokers who had stopped smoking not more than 10 years before invitation to screening). Participants were randomized into an intervention arm of five annual LDCT screenings and a control arm without screening. Details about study design and mortality-reduction results from the trial have been published elsewhere (4,26). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LUSI is a clinical research study, registered at ISRCTN (International Standard Randomized Controlled Trial Number) (28). Ethical approval was provided by the University of Heidelberg Medical Ethics Committee (073/2001). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants enrolled provided written informed consent.
Nodule management protocol
Similar to the Lung Imaging Reporting and Data System (LungRADS®) assessment guidelines (29) nodules detected for the first time by LDCT in any screening round were classified by size (largest diameter) into four categories: (I) no nodules or less than 5 mm, (II) 5–7 mm, (III) 8–<10 mm and (IV) 10 mm or larger. Accordingly, screening participants were: (I) returned to regular annual screening, invited for follow-up LDCT after (II) 6 months or (III) 3 months, or (IV) recommended immediate diagnostic work-up. In screening rounds 2–5, work-up of the nodules already observed in earlier screens was based exclusively on nodule growth, and classified into three categories: (I) no growth or volume doubling time (VDT) more than 600 days (returned to regular annual screening), (II) VDT within 400–600 days (invited for LDCT after 6 months) or (III) VDT 400 days or less (recommended immediate work-up). A schematic presentation of the nodule management protocol used in LUSI is given in Table S1. For immediate work-up, participants were referred to a cooperating pulmonologist, who then decided about further diagnostic procedures or treatments [X-ray, full-dose computed tomography (CT), positron emission tomography (PET), bronchoscopy, video-assisted thoracoscopic surgery (VATS), biopsy, antibiotic treatment and short-term follow-up].
Blood sample collection protocol
Among the 2,029 participants assigned to the LDCT arm, a total of 1,576 provided a blood sample at first screening participation [Round 1 (T0); N=1,362] or on a subsequent screening round (206 in round 2; 8 in rounds 3 to 5). Beyond this baseline sample collection, further samples were collected from individuals who, at any screening round, presented with suspicious LDCT scan results. This included all study participants who were referred to the local hospital clinic for immediate confirmatory diagnostic work-up (N=111), plus those returning for 3-month (N=132) or 6-month (N=408) follow-up examinations by LDCT to determine whether or not a suspicious pulmonary nodule was fast-growing. All blood samples were processed within 2 hours of the blood draw. Serum was allowed to clot for 30 minutes followed by centrifugation and aliquoting before long term storage at −80 °C. A detailed overview of blood samples collected at baseline and at follow-up examinations is in Table S2.
CT-detected lung cancer cases
In the LDCT arm, a total of 69 lung cancer cases were observed during the active screening period; 63 of these were screen-detected and 6 were interval cancers (25,26). For all lung cancer cases, detailed information from medical records (pathology reports, medical letters from responsible physicians on diagnosis and treatment and radiology reports) was obtained by contacting the treating clinics and coded according to the International Classification of Diseases for Oncology, version 3 (ICD-O-3). The Supplemental study design and methods section provides further details on ICD-O-3 coding for tumor histology and stage.
Participant selection within the LUSI trial: nested case-control design
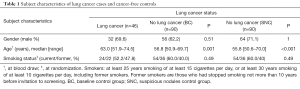
From amongst the 63 screen-detected lung cancer cases, we retrospectively selected all available blood samples taken at the time of the LDCT scan that led to further diagnostic work-up (X-ray, full-dose CT, PET, bronchoscopy, VATS, biopsy, antibiotic treatment and short-term follow-up) (n=46). Two sets of controls were selected. The first was a random selection of participants who had remained cancer-free until the end of follow up (April 30th 2019), and who provided a baseline blood sample [baseline control group (BC), n=90]. The second set was a random selection of participants returning for follow-up scans of suspicious nodules found during the screening period but in the following were not diagnosed with lung cancer [suspicious nodule control group (SNC), n=90] (Tables 1,2, Figure 1).
Full table
Full table
Sample processing and laboratory assays
Autoantibodies to seven tumor-associated antigens (CAGE, GBU4-5, HuD, MAGEA4, NY-ESO-1, p53, SOX2) were measured with the EarlyCDT®-Lung enzyme-linked immunosorbent assay (ELISA) kit (Oncimmune Ltd., Nottingham, United Kingdom), at the German Cancer Research Center, Heidelberg (immunoassay laboratory, division of cancer epidemiology). Measurements were made blinded with regards to any additional clinical information about the participants from which the samples were taken. All assays were performed on serum samples, thawed for the first time for the present study, and using a two-plate set-up, according to the manufacturer’s protocol instructions (see Supplemental study design and methods section).
EarlyCDT®-Lung results interpretation and application
EarlyCDT®-Lung classifies test results using a proprietary scoring algorithm and autoantibody-specific cut-off values. Positive results are reported as: “Moderate Level” [M] if the levels of one or more autoantibodies in the EarlyCDT®-Lung panel are above the low cut-off value but all are below the high cut-off value, as “High Level” [H] if the levels of one or more autoantibodies are above the high cut-off value, or as “No Significant Level” [NS] if the levels of autoantibodies in the panel are below the low cut-off value.
Based on studies for lung cancer detection among patients with incidentally observed pulmonary nodules in clinical settings (14,22), Oncimmune recommends combining the test result with the estimated risk of a given nodule being malignant from a model based on age, sex and radiologic (CT) data, as developed in the Mayo clinic (30). The EarlyCDT®-Lung test is then used to update the risk of patients presenting with indeterminate nodules, i.e., those with 10–65% risk of being malignant. Further diagnostic work-up is recommended for patients having ≥10% malignancy risk according to the Mayo model and with a “High Level” EarlyCDT®-Lung results, and for patients with an estimated risk ≥45% and a “Moderate Level” test result. Any other result, including “NS”, should not affect the clinical management plan. However, so far equivalent guidelines for the use of EarlyCDT®-Lung in population screening settings, and in particular, when EarlyCDT®-Lung is used as a case-finding method prior to further CT scanning, have not been issued. Thus, for our main analyses, “High” or “Moderate” level results were considered positive tests, and “NS” results were considered negative, according to the manufacturer’s indications. This classification led to an approximate 90% specificity/40% sensitivity scenario observed in several clinical studies (14,21-23). For comparison purposes, analyses were also conducted for the alternative classification, defining only “High” levels as positive and “Moderate” or “NS” as negative.
Statistical analyses
The malignancy detection sensitivity of EarlyCDT®-Lung was assessed in the set of individuals whose lung cancer was LDCT-detected. Additionally, its specificity was assessed in each of the two control groups. The positive likelihood ratio (LR+) was calculated based on estimated sensitivity and specificity. Exact binomial confidence limits were calculated for sensitivity, specificity and LR+. Logistic regression analysis was performed to assess associations of EarlyCDT®-Lung test results with case-control status, and complementary statistical tests were also performed to assess whether positive test results were related to tumor size, stage or histology. The degree of association between test results and malignancy was also evaluated among lung cancer patients showing nodules on their CT-scans and the SNC group, overall and by categories of nodule size. For (non-normally distributed) continuous variables, medians and ranges (or medians and IQR) were reported (age, tumor diameter) and differences on their central tendency parameters between groups were tested via the non-parametric Kruskal-Wallis Rank Sum Test (Mann-Whitney-U-Test when comparing two groups). The Chi-Squared test or Fisher’s exact test were applied whenever testing for differences in distributions of categorical variables as appropriate, depending on cell counts.
All analyses were carried out using the R software for statistical computing version 3.3.3 (31) and the epiR package (32).
Results
At time of blood collection, the 46 participants eventually diagnosed with lung cancer (32 of them males) were significantly older [median: 63.0 years (range, 51.9–74.5 years)] than those in both the BC group [56.8 years (range, 50.9–69.7 years), P<0.001] and the SNC group [55.8 years (range, 50.6–70.0 years), P<0.001] (Table 1).
Lung cancer detection occurred on the first (“prevalence”) screening round for 19 (41.3%) cases and on subsequent second to fifth (“incidence”) rounds for 27 (58.7%) cases. As described in the nodule management protocol, all tumors detected in the first screening round were deemed suspicious based exclusively on their size. For one of the participants, lung cancer was detected on the second screening round in the absence of pulmonary nodules, due to the identification of atelectasis in the scan images. Twenty-one (80.8%) of the remaining 26 detections in the incidence rounds were done in known nodules already observed in previous screening rounds; with 7 of these immediate recall decisions based solely on nodule volume doubling time (VDT).
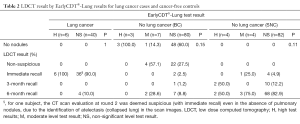
EarlyCDT®-Lung produced “High Level” test results for 6 out of the 46 CT-detected lung cancer patients. There were no “Moderate Level” test results (Table 2). This resulted in a detection sensitivity of 13.0% (95% CI: 4.9–26.3%). Within the subset of participants with nodules <10 mm in diameter, the test produced “High Level” results for 1 out of 11 CT-detected lung cancer patients, yielding a sensitivity of 9.1% (95% CI: 0.23–41.3%). For participants with nodules ≥10 mm, the estimated sensitivity was 14.7% (95% CI: 4.9–31.1%).
When considering both “High Level” and “Moderate Level” [H/M] results as positive, the false-positive detection rates were 11.1% in the BC group [specificity of 88.9% (95% CI: 80.5–94.5%)] and 8.9% [specificity of 91.1% (95% CI: 83.2–96.1%)] in the SNC group. Based on these estimates, the LR+ was then calculated at 1.17 (95% CI: 0.46–3.03) in the BC group and at 1.47 (95% CI: 0.54–3.98) in the SNC group. Logistic regression provided insufficient evidence to claim an increased risk of malignancy, regardless of which control group was considered [odds ratio (OR): 1.20 (95% CI: 0.41–3.54), P=0.74 for BC; OR: 1.54 (95% CI: 0.5–4.73), P=0.45 for SNC].
If only “High Level” results were considered positive, the false-positive detection rate was 3.3% [specificity of 96.7% (95% CI: 90.6–99.3%)] in the BC group and 4.4% [specificity of 95.6% (95% CI: 89.0–98.8%)] in the SNC group, yielding estimates of LR+ of 3.91 (95% CI: 1.03–14.94) and 2.93 (95% CI: 0.87–9.88), respectively. In this case, logistic regression showed a moderate, but significant association of test-positivity with malignancy in comparison to the BC group [OR: 4.35 (95% CI: 1.04–18.28), P=0.04] and not enough evidence of association in the SNC group [OR: 3.22 (95% CI: 0.86–12.07), P=0.08].
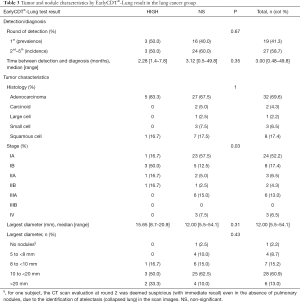
Among lung cancer patients showing nodules on their CT-Scans (N=45) (Table 3) and the SNC group, we couldn’t find enough evidence between test results and malignancy, with OR of 1.58 (95% CI: 0.51–4.86) and 3.31 (95% CI: 0.88–12.39) depending on the definition of a positive test (Table S3). Similar results were obtained when stratifying by nodule size (<10 mm, ≥10 mm). Regarding positive test results and malignancy, there was only weak evidence of association when considering only “High Level” results as significant with LR+: 1.92 (95% CI: 1.09–3.40) overall, and among participants with nodules ≥10 mm [1.17 (95% CI: 1.02–1.35)], but not among those with nodules <10 mm [OR: 1.93 (95% CI: 0.24–15.77)].
Full table
EarlyCDT®-Lung returned positive results for 5 of 6 tumors stages IB or higher (sensitivity of 22.7%), against only 1 out of 24 stage IA tumors (sensitivity of 4.2%). A “High Level” test result was associated with a significant shift in tumor stage distribution (P=0.03) towards moderately higher stages (83.3% in stages IB and above), compared to patients with negative results (NS) who were predominantly (57.5%) stage IA (Table 3). By contrast, we observed no significant association of test results with malignant nodule size or histology (Table 3).
Discussion
EarlyCDT®-Lung is currently being tested as a first-line population screening tool in a Scottish trial (N=12,209) for the identification of subjects likely to harbor a lung tumor, who are then further examined by LDCT (33). In addition, EarlyCDT®-Lung has been evaluated as a confirmatory test in clinical settings, with the purpose of deciding whether a biopsy or surgical intervention for definitive diagnosis is necessary for subjects presenting with incidentally observed pulmonary nodules (14,22,23).
After analyzing blood samples collected as part of a lung cancer screening trial, we found EarlyCDT®-Lung to have limited sensitivity for the identification of patients with lung tumors detected via LDCT. Although statistically significant associations with case-control status (and among cases with tumor stage) indicate a degree of intrinsic validity of EarlyCDT®-Lung as a tumor detection test, the test’s sensitivity [13.0% (95% CI: 4.9–26.3%)] was found to be too low for it to be of practical diagnostic use in screening context.
In the context of population screening, the Early detection of Cancer of the Lung Scotland (ECLS) study (15,34) reports a positive EarlyCDT®-Lung detection signal for 12 out of 23 cancer patients in stages I or II [sensitivity of 52.2% (95% CI: 30.6–73.2%)] and for 6 out of 33 patients with stage II–IV tumors [sensitivity of 18.2% (95% CI: 7.0–35.5%)]. The ECLS also found a statistically significant shift towards earlier stages for tumors identified by EarlyCDT®-Lung, later confirmed by standard chest X-ray and LDCT, among 12,208 men and women at elevated risk (1–2%) of developing lung cancer within 24 months (50–75 years of age, smokers and ex-smokers with a close relative with lung cancer). In contrast, our findings suggest that EarlyCDT®-Lung would miss the majority of early-stage small tumors that can be detected by LDCT. This puts into question whether multi-modal screening with EarlyCDT®-Lung as a pre-CT screening tool can reduce lung cancer mortality to a similar degree as observed in CT-based screening trials.
As a test for further diagnostic triage after LDCT screening, for individuals with screen-detected indeterminate pulmonary nodules, our data indicate that EarlyCDT®-Lung may also have insufficient sensitivity to reliably identify patients for whom more invasive diagnostics involving biopsy or surgery should be recommended. As mentioned in the Methods section, the EarlyCDT® test is being proposed as a “rule-in” test to identify patients with increased risk of having a pulmonary malignancy, whereas a negative EarlyCDT® test should not affect the clinical management plan; that is, EarlyCDT® is not meant to be used as a “rule-out” test. The low sensitivity of the test as estimated from our data confirms the recommendations from the providers. Similarly, previous studies (14,22,23) among clinical case series of patients with indeterminate nodules (22) indicated that the negative predictive value (NPV) of the EarlyCDT®-Lung may be too low for it to be used reliably for exclusion of individuals from further, more invasive diagnostics such as biopsies, although they did report higher sensitivities for EarlyCDT®-Lung as a detection test.
Regarding the association between a positive test result and malignancy among subjects with suspicious nodules on their CT-scan images, our positive likelihood ratio estimates (1.5 to 1.9 depending on positive test definition) are in line with those from Massion et al. [LR+: 2.3 (95% CI: 1.3–4.1), all nodules combined) (14,23). Our results, however, were not statistically significant which might be attributed to the small sample size of this study. Our study is the first to evaluate the discrimination performance of the EarlyCDT®-Lung in the context of LDCT-based screening, with blood samples available at baseline CT screening appointments as well as for individuals undergoing accelerated (3- or 6-month) follow-up screenings because of suspicious CT-findings. A strength of this study is the linkage of malignancy to specific nodules. This is in contrast to previous studies, in which participants were represented by the largest non-calcified nodule observed (14,23). A limitation of our study is that, given the blood collection protocol, no samples are available from participants with interval cancers (n=6) who, by definition, received a lung cancer diagnosis without previous suspicious findings on their CT-scan appointments. Without such samples, we are unable to evaluate whether malignancy that went undetected by CT could have been detected by the test. Independently of the latter limitation, however, our findings do indicate that the EarlyCDT®-Lung TAAb panel—a test previously shown to provide a potential decision aid for further diagnostic work-up or treatment of clinical patients with suspected lung cancer—may not be sensitive enough to detect cancer at stages detectable by LDCT in a screening setting.
Acknowledgments
We are much obliged to many colleagues who contributed by their engagement to the success of this study: in the years 2007–2011, M.-L. Gross carried out recruitment with initial patient information and randomization. K. Lenner-Fertig worked-up the blood samples and organized the freezer storage. A. Albrecht and U. Beckhaus mailed the annual questionnaires, performed the scanning of the filled-in questionnaires and data entry into the database, and kept telephone contact in case of doubtful answers or missing feedback. A. Trotter performed data management throughout the trial. The LDCTs were performed by J. Engelhardt and M. Jochim. We would also like to thank J. Tremper, M. Eichinger, D.E. Optazaite, M. Puderbach and M. Wielpütz for radiologic evaluations of the LDCTs. Particular thanks go to all study participants who beautifully complied with the study protocol and thus carried the study to its success. The LUSI trial was originally initiated by N. Becker, German Cancer Center Heidelberg, who served as PI of the project till December 2018. R. Kaaks had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by a grant from the German Center for Lung Research (DZL) [82DZL00404 to R.K.]. The LUSI Trial was supported by funding from the Dietmar Hopp-Foundation together with the German Research Foundation [BE 2486/2-1] in the years 2007-2010, and from the German Research Foundation [BE 2486/2-2] in the years 2010-2013.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-727
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-727
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-727
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-727). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LUSI is a clinical research study, registered at International Standard Randomized Controlled Trials Number (ISRCTN). Ethical approval for the LUSI trial was given by the University of Heidelberg Medical Ethics Committee (073/2001). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants enrolled provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Paci E, Puliti D, Lopes Pegna A, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017;72:825-31. [Crossref] [PubMed]
- Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. J Cancer Res Clin Oncol 2012;138:1475-86. [Crossref] [PubMed]
- Pastorino U, Silva M, Sestini S, et al. Prolonged Lung Cancer Screening Reduced 10-year Mortality in the MILD Trial. Ann Oncol 2019;30:1672. [Crossref] [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. [Crossref] [PubMed]
- Mazzone PJ, Silvestri GA, Patel S, et al. Screening for Lung Cancer: CHEST Guideline and Expert Panel Report. Chest 2018;153:954-85. [Crossref] [PubMed]
- Chu GCW, Lazare K, Sullivan F. Serum and blood based biomarkers for lung cancer screening: a systematic review. BMC Cancer 2018;18:181. [Crossref] [PubMed]
- Hasan N, Kumar R, Kavuru MS. Lung cancer screening beyond low-dose computed tomography: the role of novel biomarkers. Lung 2014;192:639-48. [Crossref] [PubMed]
- Hulbert A, Jusue-Torres I, Stark A, et al. Early Detection of Lung Cancer Using DNA Promoter Hypermethylation in Plasma and Sputum. Clin Cancer Res 2017;23:1998-2005. [Crossref] [PubMed]
- Lam S, Boyle P, Healey GF, et al. EarlyCDT-Lung: an immunobiomarker test as an aid to early detection of lung cancer. Cancer Prev Res (Phila) 2011;4:1126-34. [Crossref] [PubMed]
- Liang W, Zhao Y, Huang W, et al. Liquid biopsy for early stage lung cancer. J Thorac Dis 2018;10:S876-81. [Crossref] [PubMed]
- López-Sánchez LM, Jurado-Gamez B, Feu-Collado N, et al. Exhaled breath condensate biomarkers for the early diagnosis of lung cancer using proteomics. Am J Physiol Lung Cell Mol Physiol 2017;313:L664-76. [Crossref] [PubMed]
- Massion PP, Healey GF, Peek LJ, et al. Autoantibody Signature Enhances the Positive Predictive Power of Computed Tomography and Nodule-Based Risk Models for Detection of Lung Cancer. J Thorac Oncol 2017;12:578-84. [Crossref] [PubMed]
- Sullivan FM, Farmer E, Mair FS, et al. Detection in blood of autoantibodies to tumour antigens as a case-finding method in lung cancer using the EarlyCDT(R)-Lung Test (ECLS): study protocol for a randomized controlled trial. BMC Cancer 2017;17:187. [Crossref] [PubMed]
- Chapman CJ, Murray A, McElveen JE, et al. Autoantibodies in lung cancer: possibilities for early detection and subsequent cure. Thorax 2008;63:228-33. [Crossref] [PubMed]
- Broodman I, Lindemans J, van Sten J, et al. Serum Protein Markers for the Early Detection of Lung Cancer: A Focus on Autoantibodies. J Proteome Res 2017;16:3-13. [Crossref] [PubMed]
- Du Q, Yu R, Wang H, et al. Significance of tumor-associated autoantibodies in the early diagnosis of lung cancer. Clin Respir J 2018;12:2020-8. [Crossref] [PubMed]
- Qin J, Zeng N, Yang T, et al. Diagnostic Value of Autoantibodies in Lung Cancer: a Systematic Review and Meta-Analysis. Cell Physiol Biochem 2018;51:2631-46. [Crossref] [PubMed]
- Yang B, Li X, Ren T, et al. Autoantibodies as diagnostic biomarkers for lung cancer: A systematic review. Cell Death Discov 2019;5:126. [Crossref] [PubMed]
- Chapman CJ, Healey GF, Murray A, et al. EarlyCDT(R)-Lung test: improved clinical utility through additional autoantibody assays. Tumour Biol 2012;33:1319-26. [Crossref] [PubMed]
- Jett JR, Peek LJ, Fredericks L, et al. Audit of the autoantibody test, EarlyCDT(R)-lung, in 1600 patients: an evaluation of its performance in routine clinical practice. Lung Cancer 2014;83:51-5. [Crossref] [PubMed]
- Healey GF, MacDonald IK, Reynolds C, et al. Tumor-Associated Autoantibodies: Re-Optimization of EarlyCDT-Lung Diagnostic Performance and Its Application to Indeterminate Pulmonary Nodules. J Cancer Ther 2017;8:506-17. [Crossref]
- Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res 2005;4:1123-33. [Crossref] [PubMed]
- González Maldonado S, Delorme S, Husing A, et al. Evaluation of Prediction Models for Identifying Malignancy in Pulmonary Nodules Detected via Low-Dose Computed Tomography. JAMA Netw Open 2020;3:e1921221. [Crossref] [PubMed]
- Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening - results from the randomised German LUSI trial. Int J Cancer 2020;146:1503-13. [Crossref] [PubMed]
- Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [Crossref] [PubMed]
- ISRCTN30604390. Spiral computed tomography scanning for the early detection of lung cancer 2007 [Trial website]. Available online: http://www.isrctn.com/ISRCTN30604390
- Lung RADS. Lung CT Screening Reporting & Data System (Lung-RADS). Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [Crossref] [PubMed]
- R Core Team. The R package for statistical computing: R: A language and environment for statistical computing. R Foundation for Statistical Computing ed. Vienna, Austria, 2013. Available online: https://www.R-project.org/
- Stevenson M, Sergeant E. R Package epiR: Tools for the Analysis of Epidemiological Data. 1.0-14 ed. Available online: ; 2020.https://CRAN.R-project.org/package=epiR
- Sullivan FM, Mair FS, Anderson W, et al. Earlier diagnosis of lung cancer in a randomised trial of an autoantibody blood test followed by imaging. Eur Resp J 2020. [Epub ahead of print]. doi: 10.1183/13993003.00670-2020. [Crossref]
- Sullivan F, Schembri S. Early detection of cancer of the lung Scotland (ECLS): trial results. J Thorac Oncol 2019;14:S5. [Crossref]



