
Sleeve lobectomy after neoadjuvant chemoimmunotherapy/chemotherapy for local advanced non-small cell lung cancer
Introduction
Bronchial and/or pulmonary arterial sleeve lobectomy was initially developed for non-small cell lung cancer (NSCLC) patients with insufficient pulmonary reserve (1). Sleeve lobectomy is now widely accepted as a reliable and safe procedure to allow complete resection of tumors invading the central structures, which offers better short-term recovery outcomes and long-term survival outcomes than pneumonectomy (2).
When considering patients with locally advanced NSCLC, neoadjuvant chemotherapy or chemoradiotherapy were traditionally the standardized indications, especially in the presence of N2 disease (3). However, the development of PD-1/PD-L1 check-point inhibitors has changed the therapy pattern for local advanced NSCLC. The NADIM study was the first to evaluate the potential therapeutic effect of neoadjuvant PD-1 inhibitors in combination with chemotherapy in stage IIIA NSCLC patients (4). A high major pathological response (MPR) rate of 85.36% and 100% R0 resection rate hint the combination neoadjuvant strategy might be a new option for local advanced NSCLC. However, concerns of additional perioperative risks with this approach include increased difficulty in surgical resection caused by diffuse fibrotic reaction, tissue edema, lack of interstitial space and the potential healing impairment of the reconstructed bronchus caused by tissue damage and compromised vascularization (5).
Sleeve lobectomy following neoadjuvant chemotherapy or chemoradiotherapy has recently been reported as a safe procedure (6). Our team has also reported similar results (7). However, no study to date has ever explored whether neoadjuvant chemoimmunotherapy (IO+C) would increase surgical risk or postoperative complications in followed sleeve lobectomy. The aim of this study was to evaluate the surgical, oncological and perioperative outcomes of neoadjuvant IO+C for the treatment of local advanced NSCLC in patients who underwent subsequent sleeve lobectomy. We present the following article in accordance with the strengthening the reporting of observational studies in epidemiology (STROBE) guideline checklist (8) (available at http://dx.doi.org/10.21037/tlcr-20-778).
Methods
Study design and patient inclusion
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol and methods were reviewed by the institutional ethics committee of the First Affiliated Hospital of Guangzhou Medical University (2020-69). The data of all biopsy-verified NSCLC patients after sleeve lobectomy consecutively admitted to the First Affiliated Hospital of Guangzhou Medical University between December 2017 and January 2020 were retrospectively collected. We only included local advanced NSCLC patients who received neoadjuvant therapy. Neoadjuvant therapy was given when the disease was unresectable due to bulky N2, multi-station N2 or invasion of critical structures. The neoadjuvant strategy included chemotherapy or chemotherapy in combination with PD-1 inhibitors (IO+C). Prior to the availability of PD-1 inhibitors in China (2018), neoadjuvant chemotherapy was used. After approval of PD-1 inhibitors by the China Food and Drug Administration (CFDA), neoadjuvant IO+C were considered priority. Informed consents were obtained from every patient before the neoadjuvant procedure.
Computed tomography (CT) scan of all patients was performed to confirm the size and position of lesions by two independent radiologists before the first cycle of neoadjuvant therapy. Brain magnetic resonance imaging (MRI), bone scan or positron emission tomography (PET) examination were performed to exclude distant metastasis. Preoperative lymph node status was assessed via one of following techniques: contrast-enhanced CT, PET, endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) or mediastinoscopy. Not all patients underwent PET examination or invasive mediastinal staging pre-operatively. Except for necessary CT scan and physical examination, all other evaluation strategies would be discussed and decided together with patients and his/her family members.
Neoadjuvant strategy and surgical technique
Biopsies via CT-guided percutaneous or EBUS-TBNA were obtained to confirm the histology types before neoadjuvant therapy. A PD-L1 expression test was used but not necessary in every case. Platinum-based doublet chemotherapy was prescribed every 21 days (3), with or without PD-1 inhibitors for the IO+C and chemotherapy groups, respectively. The strategy for neoadjuvant therapy was decided and agreed upon together by a group oncologists, surgeons and patients. CT scan was used to re-evaluate the lesion every 2 cycles of neoadjuvant therapy. Importantly, the neoadjuvant cycle was not fixed, as the surgery was performed at a time the surgeons regarded radical resection could best be accomplished.
Resection was initially performed under video-assisted thoracoscopic surgery (VATS), and conversion to hybrid VATS or open surgery was conducted when necessary. Sleeve lobectomy comprises lobectomy with complete circular bronchus sleeve resection and systematic hilar and mediastinal lymphadenectomy. The same procedure of lymphadenectomy was performed in both groups. Angioplasty or intrapericardial resections were added when necessary. The bronchial and vessel endings were adapted end-to-end using a 3-0 and 5-0 prolene running suture, respectively. The anastomosis was covered by the interposition of the vascular pedicled thymic flap, prepericardial fat, thymus or mediastinal pleura in selected cases.
Data collection and evaluation
Data were extracted independently by two investigators and conflicts were adjudicated by a third investigator. The following outcomes were used to make the comparison between neoadjuvant chemotherapy vs. IO+C: (I) intraoperative parameters: operating time, bleeding volume, conversion rate, lymph node or stations retrieved; (II) postoperative recovery outcomes: 30-day mortality, morbidity, duration of hospitalization, drainage volume and days of tube removal; (III) oncological response evaluation: radiological and pathological regression; (IV) baseline details.
The tumor radiologic response was evaluated through Response Evaluation Criteria for Solid Tumors version 1.1 (RECIST 1.1) (9). Pre-therapy, post-adjuvant therapy and postoperative staging was evaluated in accordance with the 8th American Joint Committee on Cancer (AJCC) lung cancer staging manuals on tumor, lymph node and metastasis (TNM) staging systems (10). Radiologic-regression rate (RRR) of tumor was defined as the longest diameter of the tumor after neoadjuvant treatment, divided by the longest diameter of the tumor before neoadjuvant treatment.
Pathological analyses were performed on available biospecimens of surgical groups by two senior pathologists. Hematoxylin-eosin staining was performed for all paraffin sections. MPR rate was defined as 10% or less viable tumor remaining on postoperative pathologic review, which was identified on routine hematoxylin and eosin staining (11,12). No residual tumor cells found in dissected tissues and lymph nodes was defined as complete pathological response (CPR) (13). The IO+C group have the same pathological evaluation procedure with the chemotherapy group. The pathological evaluation for lymph node is also the same with that of primary tumor.
Statistical analyses
Continuous data are presented as mean and standard deviation and were analyzed with 2-sample Student’s t-tests for independent data. Categorical variables are given as a count and percentage of patients and compared with the χ2 or Fisher’s exact test. Patients were followed up by Oct. 1st, 2020. Progression-free survival (PFS) and overall survival (OS) were calculated. Kaplan-Meier (K-M) method was used to evaluate the survival status between two groups and compared with the log-rank test. Hazard ratios (HRs) were calculated with 95% confidence intervals (CIs). All tests were two-sided, with an α-level of 0.05. SPSS software (SPSS version 25.0; IBM Corp, Armonk, NY, USA) and GraphPad Prism 6.0 were used for all statistical evaluations.
Results
Patients characteristics
A total of 108 patients between December 2016 and December 2019 that underwent sleeve lobectomy were retrospectively included in this analysis, of which 91 were local advanced NSCLC patients (Figure 1). Among them, 10 patients received neoadjuvant chemotherapy (11%) and 10 patients were treated with neoadjuvant IO+C (11%). The baseline characteristics are summarized in Table 1. Of the 20 patients, 2 were staging at IIb (10%), 15 were IIIa (75%) and 3 were IIIb (15%). Importantly, these baseline demographics and clinical variables were well balanced between the two groups (Table 1).
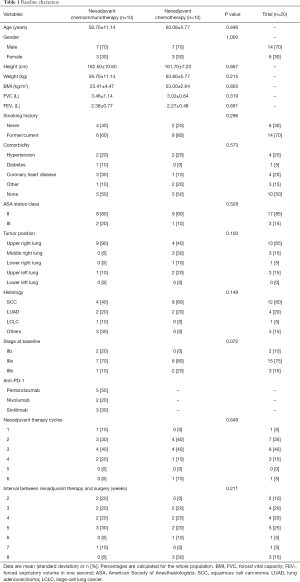
Full table
Efficacy of neoadjuvant therapy
No patients died or experienced severe side effects during neoadjuvant therapy in either group. According to imaging evaluation before and after neoadjuvant therapy, the average RRR was 35.78% and 20.86% in the neoadjuvant IO+C and chemotherapy groups (P=0.416), respectively. No radiological complete response (CR) occurred in either group. In the neoadjuvant IO+C group, 8/10 (80%) patients achieved partial response (PR), and no progression of disease (PD) occurred. Conversely, in the neoadjuvant chemotherapy group, only 3/10 (30%) patients achieved PR, while PD occurred in 2/10 (20%) of patients.
After surgical resection, 1/10 (10%) patients achieved CPR and 5/10 (50%) patients achieved MPR in the neoadjuvant IO+C group, while only 3/10 (30%) patients achieved MPR in the neoadjuvant chemotherapy group. Interestingly, postoperative histological evaluation indicated that no tumor cells were found in the lymph nodes (N1 or N2) of 7/10 (70%) patients in the neoadjuvant IO+C group, as opposed to only 3/10 (30%) patients in neoadjuvant chemotherapy group.
Re-staging according to 8th AJCC was conducted after surgery. The down-staging rate was 8/10 (80%) in the neoadjuvant IO+C group and 6/10 (60%) in the neoadjuvant chemotherapy group. However, 2/10 (20%) patients had up-staging in the neoadjuvant chemotherapy group (Figure 2). According to preoperative imaging evaluation, these 2 patients corresponded to the 2 PD cases.
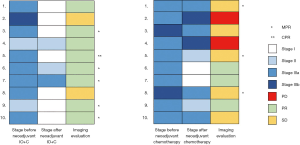
The tumor and lymph node outcomes after surgery are summarized in Table 2. Less N1 (average number 4.70 vs. 7.40) and N2 (average number 9.80 vs. 20.10) lymph nodes were acquired in the neoadjuvant IO+C group than the neoadjuvant chemotherapy group. The number of lymph nodes positive for tumor cells was also less in the neoadjuvant IO+C group than the neoadjuvant chemotherapy group, both in N1 (0.40 vs. 1.60) and N2 (0.10 vs. 1.30). Although not statistically significant, the positive lymph node ratio (LNR) was lower in the neoadjuvant IO+C group, both in N1 (0.05 vs. 0.15) and N2 (0.01 vs. 0.09). However, 8/10 (80%) and 9/10 (90%) patients were negative for tumor cells in both N1 and N2 after neoadjuvant IO+C, while only 6/10 (60%) and 5/10 (50%) patients showed negative N1 and N2 disease in neoadjuvant chemotherapy.

Full table
Intraoperative outcomes and postoperative recovery
There were 2 cases that underwent double sleeve lobectomy of bronchoplasty and pulmonary arterial angioplasty (1 in neoadjuvant IO+C group and 1 in neoadjuvant chemotherapy group), 1 case that underwent autogenous lobar transplantation in the neoadjuvant IO+C group and 1 case that underwent double sleeve lobectomy (plasty of bronchus, pulmonary artery) and superior vena cava reconstruction in the neoadjuvant chemotherapy group. Other cases were performed with bronchoplasty.
No surgery-related deaths or conversion to open surgery occurred. Of all patients where VATS was initially performed, 3/10 (30%) patients in the neoadjuvant IO+C group and 1/10 (10%) patients in the neoadjuvant chemotherapy group were converted to the hybrid VATS with a 10-cm operating incision due to pleural adhesions. Similar bleeding volume (365.00 vs. 347.50 mL; P=0.267) between the neoadjuvant IO+C and neoadjuvant chemotherapy groups was observed. Operation time (291.88 vs. 287.50 min; P=0.886) was also similar between the two groups (Table 3).
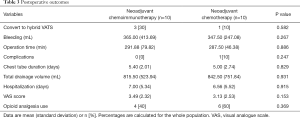
Full table
No incidence of post-operative complication was found in neoadjuvant IO+C group, while 1 incident of chylothorax occurred in the neoadjuvant chemotherapy group. Chest tube duration (5.40 vs. 5.00 days; P=0.829) and total drainage volume (815.50 vs. 842.50 mL; P=0.931) were similar in the neoadjuvant IO+C and neoadjuvant chemotherapy groups. The length of hospital-stay (7.00 vs. 6.56 days; P=0.915) was also similar between two groups. Bronchoscopy examination indicated that all patients suffering bronchus anastomosis recovered well up to 1 month after surgery (Table 3).
Postoperative pain evaluation
A similar level of visual analogue scale (VAS) score was observed in neoadjuvant IO+C compared with the neoadjuvant chemotherapy group (3.49 vs. 3.13, P=0.153) (Table 3). In addition, the number of patients using postoperative opioid analgesia was similar in the neoadjuvant IO+C compared with the neoadjuvant chemotherapy group (4 vs. 6, P=0.369).
Histological change of tissue after neoadjuvant therapy
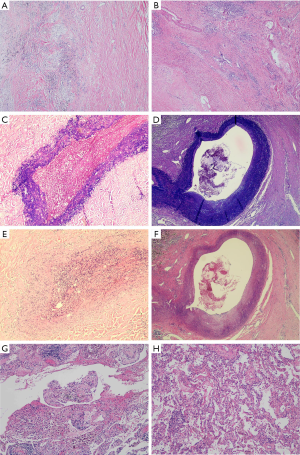
We selected two MPR patients (Figure 3A,B) from the neoadjuvant IO+C and neoadjuvant chemotherapy groups to examine the histological change of different tissues other than tumor (Table 4). Destruction of elastic fibers of the blood vessels was more severe in the neoadjuvant IO+C patients (Figure 3C,D). Vascular wall degeneration, fibrinoid necrosis and fibrosis were also more severe in neoadjuvant IO+C patients (Figure 3E,F). Furthermore, more pulmonary interstitial exudation was found in neoadjuvant IO+C patients compared to the neoadjuvant chemotherapy patients (Figure 3G,H).

Full table
Follow-up and survival outcomes of patients
Patients were followed up by Oct. 1st, 2020, and only 1 patient in neoadjuvant chemotherapy group was lost in contact. The median follow-up time were 406 and 623 days in neoadjuvant IO+C and neoadjuvant chemotherapy, respectively. All patients in neoadjuvant IO+C were alive, and no one suffered from recurrence or death. However, 4 patients in neoadjuvant chemotherapy group dead after recurrence or metastasis (Figure 4).

Discussion
In this study, we reported our initial results from 10 cases of sleeve lobectomy in patients with local advanced NSCLC after receiving neoadjuvant IO+C. Although no major complications occurred and similar intraoperative and postoperative recovery outcomes were observed compared with neoadjuvant chemotherapy, we regarded it is more difficult to perform complex reconstruction operations after pre-operative treatment added with PD-1 inhibitors.
Numerous phase I and II clinical trials have reported the oncological outcomes of NSCLC after neoadjuvant PD-1 inhibitors with or without chemotherapy. The first of these studies was CHEKMATE 159 published in 2018 (14): two preoperative doses of PD-1 inhibitor nivolumab were administered to early (stage I, II, or IIIA) NSCLC patients intravenously every 2 weeks, with surgery planned approximately 4 weeks after the first dose. An MPR occurred in 9 of 20 resected tumors (45%). Responses occurred in both PD-L1 positive and negative tumors. Additionally, the NEOSTAR study reported the first neoadjuvant doublet immunotherapy (15): three doses of nivolumab with or without ipilimumab were administered to stage I–IIIA NSCLC patients every 2 weeks. Of the 16 resectable cases in the doublet agent group, 7/16 (~44%) patients achieved MPR, a response percentage similar to that reported for single agent therapy in the CHECKMATE 159 study. However, the NADIM study was the first to explore the outcomes of PD-1 inhibitors in combination with neoadjuvant chemotherapy in stage IIIA NSCLC patients (4). In this study, they reported the highest MPR of all PD-1 neoadjuvant studies to date; 35 (85.36%) patients achieved MPR and 25 (71.4%) patients achieved CPR. These results are similar to what we found in our first-line treatment of negative mutated advanced NSCLC: the combination therapy of PD-1/PD-L1 inhibitors and chemotherapy was superior to PD-1/PD-L1 single agent, regardless of PD-L1 expression (16). In this study, the patients were all initially unresectable cases, thus the combination therapy of IO+C was given without necessarily testing PD-L1 expression in advance. We also found the MPR (including CPR) rate was 60% in the neoadjuvant IO+C group, which was double that of the group that received neoadjuvant chemotherapy alone (30%). However, limited by case number, statistically significance was not achieved. Therefore, the addition of future patient data and larger datasets will allow for a more accurate comparison between neoadjuvant IO+C and neoadjuvant chemotherapy, and the reliability of neoadjuvant IO+C in improving patient outcomes.
Some clinical trials also have reported the surgical outcomes of lung cancer after neoadjuvant immunotherapy. CHEKMATE 159 enrolled 22 patients in total, 20 of which underwent resection. There were no delays to surgical resection because of immunotherapy. Of the 13 procedures attempted via a minimal invasive approach, 7/13 (~54%) required conversion to open surgery and postoperative morbidity occurred in 10/20 patients (50%). Notably, only one sleeve lobectomy case was performed in this trial (17). Another retrospective study evaluated the use of neoadjuvant immunotherapy for unresectable or metastatic NSCLC, which also reported a high conversion to open surgery rate (25%) and a high postoperative complication (32%) after neoadjuvant immunotherapy. This study didn’t report any sleeve lobectomy cases (18). The NEOSRAR study has also reported its surgical results, 1 (3%) patient died after surgery because of bronchopleural fistula. Postoperative leak occurred in 8 (22%) patients. Surgeons who participated in the trial regarded 41% (15/37) of operations were more difficult than usual and 19% (7/37) of operations persisted over 4 hours (15). However, compared with histological data, neoadjuvant PD-1 inhibitors did not increase postoperative complications and had similar recovery outcomes compared with surgery alone.
According to current evidence, we have reason to believe the surgery after neoadjuvant IO+C would be more challenging than chemotherapy or single agent PD-1 inhibitors, especially performing the sleeve lobectomy. In this study, although postoperative recovery was similar between two group, we found it is more difficult to perform the sleeve operation after neoadjuvant IO+C. During the operation, first, the space between vessels and bronchus is very narrow and the majority of cases in IO+C group presented more severe tissue edema and increased vascular fragility, thus surgeons noted the space between vessels and tissue were difficult to divide; second, the hilar and mediastinal lymph node are tougher and more difficult to dissect from the bronchus; third, the visceral pleura becomes tighter, which is more difficult to cut with electrosurgical or ultrasound scalpel. All these factors contributed to a potentially higher risk of bleeding. The fact that we observed more severe tissue degeneration in histological paraffin sections of the neoadjuvant IO+C group than the neoadjuvant chemotherapy group lends some credence to this hypothesis. However, this opinion also needs prospective data to support. The subjective difficulty of surgery after IO+C should be evaluated by a scoring system to facilitate quantification of apparent surgical complications in future studies.
We can observe that although no statistically significant (probably owing to the sample size), the operation time (291.88 vs. 287.50 min) was longer and the intraoperative bleeding volume (365.00 vs. 347.50 mL) was more in IO+C group when the lymph node dissection number was significantly less in IO+C group. Besides, from the results of the NEOSTAR study (15), 40% of operations after immunotherapy was judged to be “more difficult” than usual cases, we have the reason to believe that operation after IO+C is more difficult than that after immunotherapy alone. However, we must acknowledge that the systematic objective evaluation of the difficulty should be prospectively applied to confirm this preliminary subjective perception.
Several limitations should be acknowledged. First, the small sample size and retrospective nature limited its statistical power and may have selection biases, however, sleeve lobectomy is not a routine procedure in clinical practice, especially after neoadjuvant IO+C. This study reported the largest cohort to announce its surgical clinical features. Second, all patients underwent neoadjuvant IO+C in 2018 or 2019, limiting the insufficient follow up time, the outcomes of PFS and OS were premature, and the prognostic impact of major and CPR is not clearly, either. Third, not all patients underwent mediastinum pathological staging, which might affect the postoperative N stage evaluation. However, this study focused on surgical outcomes independent of lymph node status. Fourth, not all patients underwent PD-L1 expression examination. According to a recent study (5), PD-L1 expression did not affect the results in neoadjuvant setting. In addition, referring from first-line treatment for advanced NSCLC, PD-1 inhibitors in combination with chemotherapy showed better efficacy than monotherapy of either immunotherapy or chemotherapy (16). Thus, PD-L1 expression test is not necessary in this study. Last, the hilar and mediastinal lymph node are tougher and more difficult to dissect from the bronchus after neoadjuvant IO+C, thus the number of harvested lymph nodes was less in IO+C group than C group, and this could be a potential bias in term of downstaging rate and post-operative complications.
Conclusions
In this analysis, we reported the initial experience of sleeve lobectomy for local advanced NSCLC treated with neoadjuvant IO+C vs. neoadjuvant chemotherapy. Although the operations become more complex, neoadjuvant IO+C did not add extra perioperative complications for sleeve lobectomy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-778
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-778
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-778
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-778). WL serves as an unpaid editorial board member of Translational Lung Cancer Research from Apr 2018 to Apr 2021. HL serves as a current section editor for this journal from Jan 2020 to Dec 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol and methods were reviewed by the institutional ethics committee of the First Affiliated Hospital of Guangzhou Medical University (2020-69). Informed consents were obtained from every patient before the neoadjuvant procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- THOMAS CP. Conservative resection of the bronchial tree. J R Coll Surg Edinb 1956;1:169-86. [PubMed]
- Cusumano G, Marra A, Lococo F, et al. Is sleeve lobectomy comparable in terms of short- and long-term results with pneumonectomy after induction therapy? A multicenter analysis. Ann Thorac Surg 2014;98:975-83. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. OA01.05 Phase II Study of Neo-Adjuvant Chemo/Immunotherapy for Resectable Stages IIIA Non-Small Cell Lung Cancer- Nadim Study-SLCG. J Thorac Oncol 2018;13:S320. [Crossref]
- Rendina EA, Venuta F, De Giacomo T, et al. Safety and efficacy of bronchovascular reconstruction after induction chemotherapy for lung cancer. J Thorac Cardiovasc Surg 1997;114:830-5; discussion 835-7. [Crossref] [PubMed]
- Kim HK, Cho JH, Choi YS, et al. Outcomes of neoadjuvant concurrent chemoradiotherapy followed by surgery for non-small-cell lung cancer with N2 disease. Lung Cancer 2016;96:56-62. [Crossref] [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5 Suppl 3:S267-73. [PubMed]
- Sharp MK, Glonti K, Hren D. Online survey about the STROBE statement highlighted diverging views about its content, purpose, and value. J Clin Epidemiol 2020;123:100-6. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Detterbeck FC, Chansky K, Groome P, et al. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1433-46. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-e50. [Crossref] [PubMed]
- Mouillet G, Monnet E, Milleron B, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol 2012;7:841-9. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. Erratum in: N Engl J Med. 2018 Nov 29;379(22):2185. doi: 10.1056/NEJMx180040. Epub 2018 Nov 9. [Crossref] [PubMed]
- Cascone T, William WN, Weissferdt A, et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. J Clin Oncol 2019;37:abstr 8504.
- Liang H, Liu Z, Cai X, et al. PD-(L)1 inhibitors vs. chemotherapy vs. their combination in front-line treatment for NSCLC: An indirect comparison. Int J Cancer 2019;145:3011-21. [Crossref] [PubMed]
- Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. [Crossref] [PubMed]
- Bott MJ, Cools-Lartigue J, Tan KS, et al. Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors. Ann Thorac Surg 2018;106:178-83. [Crossref] [PubMed]



