
Outcomes for localized treatment of large cell neuroendocrine carcinoma of the lung in the United States
Introduction
Large cell neuroendocrine carcinoma (LCNEC) comprises approximately 3% of lung malignancies in the United States. The annual age-adjusted incidence is 0.34 cases per 100,000 persons and is rising (1). About 55% of LCNEC is metastatic at time of diagnosis. Like small cell lung cancer (SCLC), LCNEC is a high-grade neuroendocrine tumor with poor prognosis and higher incidence in smokers (2). However, because of its rarity, treatment paradigms are much better established for SCLC than for LCNEC. Until recently, treatment for LCNEC had been based on extrapolation from treatment of SCLC and non-small cell lung cancer (NSCLC) or from small retrospective trials, which generally indicated some benefit from treatment with platinum-based chemotherapy (3-7).
Based on expert opinion and practice experience, for patients with early-stage disease, surgery is recommended as initial treatment (8). Two large, recent retrospective reviews indicate a benefit to adjuvant chemotherapy in patients with early stage, particularly stage IB, LCNEC (8,9). However, neither of these studies established the optimal regimen for such therapy nor the role of RT in treatment.
A number of recent prospective trials have evaluated the effectiveness of chemotherapy regimens traditionally used in NSCLC and SCLC in stage III‒IV LCNEC. Platinum-based combination therapy, frequently involving podophyllotoxins such as etoposide or camptothecins such as irinotecan, is standard of care for patients with advanced SCLC. Two multicenter prospective studies with 44 and 49 patients with stage III or IV LCNEC evaluated the efficacy cisplatin and etoposide or irinotecan in advanced LCNEC. Both studies had poor outcomes, similar to or inferior to those seen with SCLC (10,11). A recent retrospective study from the Netherlands compared platinum-etoposide chemotherapy to traditional NSCLC regimens of platinum compounds with gemcitabine, docetaxel, paclitaxel or vinorelbine. This study found a significantly increased median survival of patients receiving NSCLC platinum based regimens (8.5 months), when compared with patients receiving SCLC platinum based regimens (6.7 months) (12).
Despite these advances, data on the use of radiotherapy in LCNEC is severely lacking. Unlike in SCLC, prophylactic cranial irradiation is generally not recommended in LCNEC due to limited data on its efficacy (13,14). However, recent studies suggest that both stereotactic and whole brain irradiation have significant efficacy in patients with LCNEC metastatic to brain (15,16). Rare malignancies, such as LCNEC, are often best studied using large scale population-based databases with long term follow up such as the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database. In this study, we used SEER data to evaluate the efficacy of surgery and RT in stage I-III LCNEC. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-374).
Methods
Data source
The SEER Program is the National Cancer Institute’s authoritative source for population-based cancer incidence and survival (17) and is considered the gold standard for cancer data collection internationally (18). It is populated with data from national cancer registries and encompasses approximately 34.6% of the United States population (17). The SEER Program is updated annually for follow-up on vital status and routinely undergoes quality-control checks. The study was conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Ethical approval was waived as all research was conducted under and in accordance with the Data-Use Agreement for the 1975–2013 SEER Research Data File (SEER ID 13907, Nov 2015). Informed consent was not necessary as no identifiable data was accessed. Data were collected and analyzed as described in previous reports (19-27).
Sample selection and coding
We queried the SEER database (November, 2015 submission 1973–2013 and November, 2015 submission 1973–2015) (28) to identify all malignant cases of LCNEC [International Classification of Diseases- (ICD-) O-3 code 8013] within the lung and bronchus (ICD-O-3 codes C34.0-C34.3 and C34.8-C34.9), American Joint Committee on Cancer (AJCC) Stages I–III, diagnosed between January 1, 2004 and December 31, 2013. Cases diagnosed at autopsy or that could have 0 days of follow-up (n=1) and cases with unknown treatment (n=28) were excluded.
The following variables were collected and coded: age at diagnosis, sex, race, marital status, insurance status, ICD-0-3histology, primary site, AJCC 6th Edition Staging, AJCC 6th Edition T, N, M staging, surgery at primary site, and radiation. In SEER, all cancer-directed treatment is recorded only if it is given as part of the initial course of treatment to destroy, modify, control, or remove cancer tissue.
Statistical analysis
All statistical analyses were carried out using the IBM SPSS Statistics software package (International Business Machines Corporation, Armonk, New York). Associations between treatment and other variables were determined using the Pearson’s chi-square test. Because our predictors of interest may be closely related to each other, we tested for and found no evidence of multicollinearity (Table S1). Univariable and multivariable analyses of both overall survival (OS) and cause-specific survival (CSS) was conducted using the Cox Proportional Hazards Ratios (HR) model. 95% confidence intervals are expressed next to corresponding hazard ratios. Tests with two-tailed P values <0.05 were considered statistically significant.
Results
Patient selection and demographics
The SEER query identified 1,523 cases of LCNEC, with 748, 177, and 598 cases of stage I, II, and III disease, respectively. The majority of the cases were in males, including 50.3% of patients with stage I disease, 55.4% of patients with stage II disease and 57.0% of patients with stage 3 disease. 85% of cases of LCNEC identified occurred in white patients, 11% occurred in black patients and 3% occurred in Asian patients. Further demographic information is listed in Table 1.

Full table
Treatment
In patients with stage I disease, surgery without radiotherapy was used in 83.8% of patients, radiotherapy was added to surgery in 5.2% of patients and radiotherapy was used alone in 6.4% of patients. In stage II disease, radiotherapy was used as adjuvant treatment more frequently (20.9%) and the percent of patients receiving radiotherapy (5.1%) or surgery (63.8%) as sole treatment declined. In stage III disease, surgery was used less often (30.8%) and most patients received radiotherapy without surgery (39.5%) or did not receive surgery or radiotherapy (29.8%) (Table 1). Chemotherapy use data are not included in the SEER database and thus was not analyzed in this study.
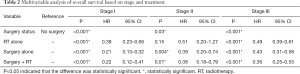
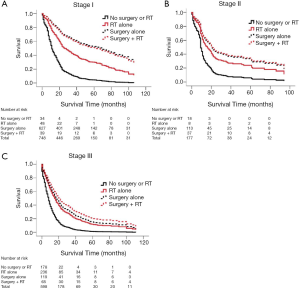
In our multivariable analysis of patients with stage I disease, surgery was the intervention most associated with favorable outcomes, with a HR of 0.21 for surgery alone (95% CI: 0.13–0.32) and 0.22 for surgery and radiation therapy (95% CI: 0.12–0.41) when compared with no intervention. Radiation alone provided significant benefit over no treatment but was inferior to surgery alone (HR 0.23, 95% CI: 0.39–0.66).
In stage II disease radiation therapy alone did not statistically improve survival over no treatment (HR 0.51, 95% CI: 0.20–1.27). Surgery alone (HR 0.39, 95% CI: 0.2–0.74) and radiation therapy in addition to surgery (HR 0.38, 95% CI: 0.18–0.79) again yielded results that were superior to no treatment but not clearly superior to surgery alone.
For patients with stage III disease, surgery combined with radiation therapy trended towards providing the best outcomes when compared with no treatment (HR 0.36, 95% CI: 0.25–0.53). Surgery alone (HR 0.43, 95% CI: 0.31–0.58) and radiation therapy alone (HR 0.49, 95% CI: 0.39–0.61) also provided significant mortality improvements over no treatment (Table 2, Figure 1). Covariables associated with treatment and survival outcomes are listed in Tables S2-S7. Regression models comparing overall and cause-specific survival (OS/CSS) yielded similar results (Table S8).
Full table
Discussion
Implications for Treatment
Our results indicate that surgery remains the mainstay of therapy in stage I and II LCENC. In patients who are not candidates for surgery or prefer nonoperative management, radiation therapy offers significant benefit in stage I disease and possibly in stage II disease. In patients with stage I and II disease who did receive surgical treatment, adding radiation therapy does not appear to confer any additional survival benefit.
Similarly, in stage I and II NSCLC radiation therapy is generally not indicated after surgical resection, except in cases of positive margins and local recurrence where it has shown some benefit (29,30). In nonsurgical candidates, radiation therapy is clearly beneficial for local treatment in NSCLC and is standard of care, with local control rates as high as 90% (31,32).
Radiation therapy has a much more established role in the treatment of limited stage SCLC with large prospective trials and meta-analyses showing significant benefits to radiation as definitive treatment in these patients (33,34). SCLC is so aggressive and radiation-responsive that prophylactic cranial irradiation is a cornerstone of treatment in many patients with SCLC (35).
Our data indicate that LCNEC outcomes with regards to surgical and radiation therapy more closely mirror those of patients with NSCLC, and NSCLC treatment paradigms with regards to RT may have utility in the treatment of LCNEC. This correlates with recent studies showing that chemotherapy regimens effective in NSCLC have considerable efficacy in LCNEC despite its neuroendocrine origins (12).
In patients with stage III disease, surgery and radiation therapy were not used in 30% of patients. However, both of these treatment modalities conferred significant benefit. Treatment with both radiation and surgery trended towards the best survival outcomes, despite being used in only 11% of patients.
Limitations
Many clinical factors that influence management decisions and survival in patients with LCNEC are not included in the SEER database, and therefore could not be controlled for in our univariable and multivariable analyses. Notable variables which could not be controlled for include patient comorbidities and treatment adequacy of radiation and surgery. This may have led to significant bias in our outcomes analyses. In particular, patients with better anticipated prognosis will often receive more aggressive treatments, including radiation and surgery, and have superior ultimate outcomes. This may lead to systemic bias that could not be adjusted for.
The decision to pursue surgery in stage III LCNEC also involves many clinical judgments such as burden of metastatic disease and tumor size and location, which were not fully reflected in the data available through SEER.
Another significant limitation is that the lack of chemotherapy data present within the SEER database. This limited our understanding of the treatment that these patients received as well as the comparisons that we were able to make. SEER also does not include data on whether patients received stereotactic body radiation therapy or conventionally fractionated radiation therapy. Finally, the overall concordance of documented radiation treatment between SEER and SEER-Medicare is 91% (36). However, the underreporting of radiation treatment in SEER would likely bias our results towards the null hypothesis, rather than exaggerate the effects size of our findings (37).
Pathologic diagnosis can be difficult to establish in patients with LCNEC, especially in those for whom only cytology or small biopsy specimens are available. We could not confirm the pathologic diagnoses recorded in the SEER database. Additionally, the co-existence of LCNEC with other subtypes of lung cancer, including SCLC and other NSCLC, is unknown. These limitations may have led to the inclusion of patients who did not have LCNEC, possibly limiting the external validity of the findings.
Directions for further study
The most important direction for future study of the efficacy of these modalities in the treatment of LCNEC are further prospective data comparing patient outcomes based on treatment approach. While our study leaves little doubt that surgery is beneficial to survival, especially in patients with early stage disease, a randomized control trial comparing surgery alone to radiation plus surgery in patients with LCNEC has the potential to be useful and ethical. This would be an especially useful question to answer in patients with positive margins or local recurrence after resection, and would allow for standardization of radiation approach across patients.
Conclusions
Due to the rarity of the disease, treatment paradigms for LCNEC are controversial and based largely upon small retrospective studies and smaller prospective trials. Our results indicate that that non-metastatic LCNEC may be treated as a NSCLC with respect to radiation. Prospective studies are necessary to increase our understanding of optimal treatment regimens for LCNEC and would be especially useful in defining the role of radiation in patients with positive margins or local recurrence after surgery.
Acknowledgments
A preliminary version of this study was accepted by the American Society of Radiation Oncology Annual Meeting 2019 (September 15–18, 2019, Chicago, Illinois) as an abstract.
Funding: The work was supported by the generous unrestricted gift from Barry Neustein and Polyflex Inc. to the lung cancer research program in Radiation Oncology at Columbia University (SKC), LUNGevity Foundation Targeted Therapeutics Award (SKC) and Louis V. Gerstner, Jr. Scholar Award (SKC).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-374
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-374). CAS receipt of personal fees from Genentech, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, outside the submitted work. TJCW reports personal fees and non-financial support from AbbVie, personal fees from AstraZeneca, personal fees from Cancer Panels, personal fees from Doximity, personal fees and non-financial support from Elekta, personal fees and non-financial support from Merck, personal fees and non-financial support from Novocure, personal fees and non-financial support from RTOG Foundation, personal fees from Rutgers, personal fees from University of Iowa, personal fees from Wolters Kluwer, grants and non-financial support from Genentech, outside the submitted work. SKC reports personal fees and non-financial support from AbbVie and Sanofi, outside of the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Ethical approval was waived as all research was conducted under and in accordance with the Data-Use Agreement for the 1975-2013 SEER Research Data File (SEER ID 13907-Nov 2015). Informed consent was not necessary as no identifiable data was accessed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kinslow CJ, May MS, Saqi A, et al. Large-Cell Neuroendocrine Carcinoma of the Lung: A Population-Based Study. Clin Lung Cancer 2020;21:e99-113. [Crossref] [PubMed]
- Fasano M, Della Corte CM, Papaccio F, et al. Pulmonary Large-Cell Neuroendocrine Carcinoma: From Epidemiology to Therapy. J Thorac Oncol 2015;10:1133-41. [Crossref] [PubMed]
- Kujtan L, Muthukumar V, Kennedy KF, et al. The Role of Systemic Therapy in the Management of Stage I Large Cell Neuroendocrine Carcinoma of the Lung. J Thorac Oncol 2018;13:707-14. [Crossref] [PubMed]
- Naidoo J, Santos-Zabala ML, Iyriboz T, et al. Large Cell Neuroendocrine Carcinoma of the Lung: Clinico-Pathologic Features, Treatment, and Outcomes. Clin Lung Cancer 2016;17:e121-9. [Crossref] [PubMed]
- Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774-85. [Crossref] [PubMed]
- Sun JM, Ahn MJ, Ahn JS, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer 2012;77:365-70. [Crossref] [PubMed]
- Yamazaki S, Sekine I, Matsuno Y, et al. Clinical responses of large cell neuroendocrine carcinoma of the lung to cisplatin-based chemotherapy. Lung Cancer 2005;49:217-23. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Evangelista A, et al. Adjuvant chemotherapy for large-cell neuroendocrine lung carcinoma: results from the European Society for Thoracic Surgeons Lung Neuroendocrine Tumours Retrospective Database. Eur J Cardiothorac Surg 2017;52:339-45. [Crossref] [PubMed]
- Raman V, Jawitz OK, Yang CJ, et al. Adjuvant Therapy for Patients with Early Large Cell Lung Neuroendocrine Cancer: A National Analysis. Ann Thorac Surg 2019;108:377-83. [Crossref] [PubMed]
- Le Treut J, Sault MC, Lena H, et al. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: the GFPC 0302 study. Ann Oncol 2013;24:1548-52. [Crossref] [PubMed]
- Niho S, Kenmotsu H, Sekine I, et al. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: a multicenter phase II study. J Thorac Oncol 2013;8:980-4. [Crossref] [PubMed]
- Derks JL, van Suylen RJ, Thunnissen E, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinomas: does the regimen matter? Eur Respir J 2017;49:1601838. [Crossref] [PubMed]
- Glisson BS, Moran CA. Large-cell neuroendocrine carcinoma: controversies in diagnosis and treatment. J Natl Compr Canc Netw 2011;9:1122-9. [Crossref] [PubMed]
- Gregor A, Cull A, Stephens RJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer 1997;33:1752-8. [Crossref] [PubMed]
- Prelaj A, Rebuzzi SE, Del Bene G, et al. Evaluation of the efficacy of cisplatin-etoposide and the role of thoracic radiotherapy and prophylactic cranial irradiation in LCNEC. ERJ Open Res 2017;3:00128-2016. [Crossref] [PubMed]
- Kawabe T, Yamamoto M, Sato Y, et al. Gamma Knife radiosurgery for brain metastases from pulmonary large cell neuroendocrine carcinoma: a Japanese multi-institutional cooperative study (JLGK1401). J Neurosurg 2016;125:11-7. [Crossref] [PubMed]
- Overview of the SEER Program. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/about/overview.html
- Davis FG, McCarthy BJ, Berger MS. Centralized databases available for describing primary brain tumor incidence, survival, and treatment: Central Brain Tumor Registry of the United States; Surveillance, Epidemiology, and End Results; and National Cancer Data Base. Neuro Oncol 1999;1:205-11. [Crossref] [PubMed]
- Boyett D, Kinslow CJ, Bruce SS, et al. Spinal location is prognostic of survival for solitary-fibrous tumor/hemangiopericytoma of the central nervous system. J Neurooncol 2019;143:457-64. [Crossref] [PubMed]
- Kinslow CJ, Bruce SS, Rae AI, et al. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: a population-based study. J Neurooncol 2018;138:173-82. [Crossref] [PubMed]
- Kinslow CJ, Rajpara RS, Wu CC, et al. Invasiveness is associated with metastasis and decreased survival in hemangiopericytoma of the central nervous system. J Neurooncol 2017;133:409-17. [Crossref] [PubMed]
- Rae AI, Mehta A, Cloney M, et al. Craniotomy and Survival for Primary Central Nervous System Lymphoma. Neurosurgery 2019;84:935-44. [Crossref] [PubMed]
- Zhou Z, Kinslow CJ, Hibshoosh H, et al. Clinical Features, Survival and Prognostic Factors of Glycogen-Rich Clear Cell Carcinoma (GRCC) of the Breast in the U.S. Population. J Clin Med 2019;8:246. [Crossref] [PubMed]
- Kinslow CJ, Garton ALA, Rae AI, et al. Extent of resection and survival for oligodendroglioma: a U.S. population-based study. J Neurooncol 2019;144:591-601. [Crossref] [PubMed]
- Kinslow CJ, May MS, Kozak M, et al. Signet ring cell carcinoma of the Ampulla of Vater: outcomes of patients in the United States. HPB (Oxford) 2020;22:1759-65. [Crossref] [PubMed]
- Kinslow CJ, Rae AI, Neugut AI, et al. Surgery plus adjuvant radiotherapy for primary central nervous system lymphoma. Br J Neurosurg 2020;34:690-6. [Crossref] [PubMed]
- Zhou Z, Kinslow CJ, Wang P, et al. Clear Cell Adenocarcinoma of the Urinary Bladder Is a Glycogen-Rich Tumor with Poorer Prognosis. J Clin Med 2020;9:138. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database. Incidence - SEER 9 Regs Research Data, Nov 2015 Sub (1973-2013)
. Available online: https://seer.cancer.gov/data-software/documentation/seerstat/nov2015/ - Wang EH, Corso CD, Rutter CE, et al. Postoperative Radiation Therapy Is Associated With Improved Overall Survival in Incompletely Resected Stage II and III Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2727-34. [Crossref] [PubMed]
- Cai XW, Xu LY, Wang L, et al. Comparative survival in patients with postresection recurrent versus newly diagnosed non-small-cell lung cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:1100-5. [Crossref] [PubMed]
- Hobbs CJ, Ko SJ, Paryani NN, et al. Stereotactic Body Radiotherapy for Medically Inoperable Stage I-II Non-Small Cell Lung Cancer: The Mayo Clinic Experience. Mayo Clin Proc Innov Qual Outcomes 2018;2:40-8. [Crossref] [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]
- Gaspar LE, Gay EG, Crawford J, et al. Limited-stage small-cell lung cancer (stages I-III): observations from the National Cancer Data Base. Clin Lung Cancer 2005;6:355-60. [Crossref] [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [Crossref] [PubMed]
- Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care 2016;54:e55-64. [Crossref] [PubMed]
- Johnson SB, Park HS, Gross CP, et al. Use of Alternative Medicine for Cancer and Its Impact on Survival. J Natl Cancer Inst 2018. [Crossref] [PubMed]


