Computer modeling of lung cancer diagnosis-to-treatment process
Introduction
Lung cancer is the second most common cancer in both men and women, accounting for about 13% of all new cancers. The American Cancer Society estimates that for year 2015, there will be about 221,200 new cases of lung cancer (115,610 in men and 105,590 in women) in the United States, with an estimated 158,040 deaths (86,380 in men and 71,660 in women) from lung cancer (1). It is the leading cause of cancer death among both men and women, accounting for 27% of all US cancer mortality (1). Survival in lung cancer mainly depends on the extent of spread (stage) at the time of treatment. The 5-year survival rate ranges from more than 60% for stage I patients, to about 40% for stage II patients. It quickly drops to 20% for stage III patients, and only 4% for stage IV patients (2). Treatment selection is also stage-dependent. Therefore, early diagnosis and staging of lung cancer is of critical importance.
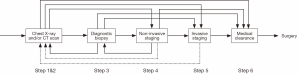
The lung cancer diagnosis process is long and complex, with substantial variations. It typically starts with an abnormal X-ray, followed by computed tomography (CT) scan and diagnostic biopsy. After radiologic (noninvasive) staging and/or invasive staging, depending on the stage, patients may be treated by surgery, chemotherapy, radiation therapy, or (as is increasingly the case) a combination of these modalities. For surgical patients, medical clearance is needed before surgery. However, different patients may follow different procedures. For instance, some patients may skip some tests, while other patients may need to go backward and repeat some tests. Figure 1 illustrates the lung cancer diagnosis process with variations for surgical patients, where the dashed lines represent unusual practices. As one can see, these variations make the diagnosis process extremely difficult to be represented using simple routes.
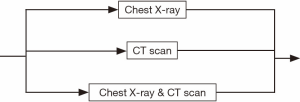
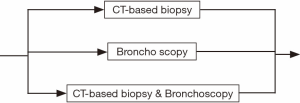
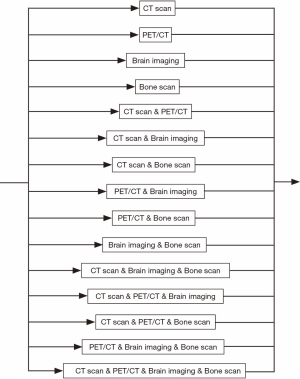
The whole diagnosis process can be divided into six steps. Within each step, the process flow can still have many variations. For instance, during the abnormal chest X-ray and/or CT scan step (see Figure 2), a patient may go to either option or both. Similarly, for the diagnostic biopsy step, the biopsy process can be carried out by major procedures, such as CT guided biopsy, bronchoscopy, or both tests may be used (see Figure 3). The non-invasive staging step is much more complex. There are more than a dozen combinations of CT scan, positron emission tomography (PET)/CT, brain imaging, and bone scan. The patient may take only one of them, or two or three of them, or even all of them (see Figure 4).
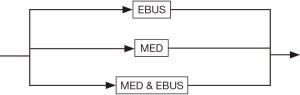
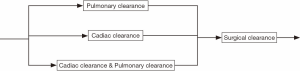
The invasive staging step is much simpler compared to non-invasive staging. The major procedures such as endobronchial ultrasound (EBUS) staging, mediastinoscopy (MED) staging, or both may be used (see Figure 5). For simplicity, we have limited the schematic to the two invasive mediastinal nodal staging procedures most commonly used in community hospitals. Finally, the medical clearance step before surgery usually includes pulmonary and cardiac clearance, each of which presumably involves visits with providers from each of these specialties. A possible combination of them provides all the variations in this process (see Figure 6). Note that the pulmonary clearance can occur well before cardiac clearance, or even before staging tests.
There can be significant waiting time between each two steps, some of which may be substantial. Although each actual test may only take a few hours, the waiting time could last from days to weeks or even months. Because of the importance of accurate staging to treatment selection and prognosis, reducing the waiting times at various steps is of significant importance. Although the importance of waiting time reduction is intuitive, how to reduce it effectively is not. First, the relationship between the waiting times between various steps, and their contribution to the overall delay time from initial lesion detection to definitive treatment is not clear. Second, although ideally all waiting times should be reduced substantially, their reduction may have different impact on the total time for diagnosis process. How to identify the most critical waiting time so that its reduction has the largest reduction of overall process time is not known. Third, how to ensure that the waiting time can be controlled within a desired time limit has not been studied. Finally, even if the mean diagnosis time is short, large variability can still lead to a substantial number of patients waiting much longer than desired. Therefore, the variance of diagnosis time plays a significant role in reducing the possibility of treatment delay. How to address the above concern in terms of variance is unknown.
To answer these questions, a detailed analysis of the lung cancer diagnosis-to-treatment process is needed. Although clinical trials can be carried out, that strategy would take an inordinate and substantial amount of effort and time. The “small tests of change” or plan-do-check-act (PDCA) model may not be either appropriate or safe in many cases. Therefore, a model based approach is needed. Computer process models can provide significant guidance to system improvement efforts, before any potentially disruptive changes in process are implemented. It can present a fresh look at the whole process, offer an alternative method to “test” changes (virtually) in practice, and evaluate the impact of those changes. In this review, we focus on two types of computer process models that can be used for this purpose: discrete event simulation (DES), and analytical modeling.
Discrete event simulation (DES) models
Literature review
Computer or discrete-event simulation has been a prevailing tool in healthcare delivery research. It has been successfully implemented in emergency departments (EDs), hospital pharmacy units, critical care units, outpatient clinics and diagnostic centers. The rapid development in information technology and data analytics has substantially enhanced the functions and efficiency of simulation tools. Using the simulation model, the practitioners can vividly emulate the events randomly happening in healthcare delivery process, test sophisticated logics and schedules, evaluate design options, assess system efficacy, and carry out ‘what-if’ analyses to investigate the complex relationships among system variables, study the impact of potential changes, and finally to provide decision support for healthcare management. By testing different scenarios of patient arrivals, staffing level, workforce and equipment configuration, bed capacity, scheduling and team policies, lab turnaround time, etc., the simulation models can help find solutions to reduce patient length of stay, increase bed utilization, identify the most critical constraints (or bottlenecks), and improve efficiency and care quality.
Comprehensive reviews of computer simulation models used in health care systems have recently been presented (3-6). In these reviews, simulation studies in multiple healthcare organizations are introduced, such as outpatient clinics, EDs, surgical centers, orthopedic departments, and pharmacies. A substantial number of studies using simulations have focused on patient flow and crowding reduction in EDs. For instance, Storrow et al. (7) discovers that reducing lab turnaround time can help reduce ED length of stay and the need for ambulance diversion. Brenner et al. (8) and Zeng et al. (9) have identified diagnostic testing as the main bottleneck in the EDs under study. Integration of registration and triage is also studied in (10). Using DES, a decision support framework is introduced in (11), which shows that in-patient bed management is the key to unblock ED outflows. In addition, Konrad et al. (12) introduces a split flow approach to bring patients to resources and providers. By verifying through DES, it shows that such an approach has the advantage to typical fast-track practice in ED.
In addition to EDs, other hospital departments and clinics also received substantial amount of research attention. For example, for intensive care units (ICUs), a simulation model is developed in (13) to determine the number of supplementary nurses in an ICU that are required to minimize overall nursing staff costs. Griffiths et al. (14) intends to optimize the number of available ICU beds in order to maintain an acceptable level of bed occupancy. Zhu et al. (15) also studies bed capacity in ICU to estimate the proper number of beds needed to meet the target service level and the extra number of beds to respond to demand growth. Azari-Rad et al. (16) studies the perioperative process in a general surgery service using simulations to reduce the number of surgical cancellations. The results indicate that scheduling surgeons on a weekly basis, sequencing surgeries in order of increasing length and variance, and adding beds to the surgical ward help reduce the number of surgical cancellations.
In pharmacies, a simulation study introduced in (17) discovered that early preparation for the returning patients and dedicating an infusion staff member for medication delivery could substantially reduce patients’ waiting time for antineoplastic medications, with up to 50% reduction achieved through such improvement efforts. Reynolds et al. (18) investigates the impact of changes in staffing levels and skill-mix on prescription workload and dispensing robot utilization in hospital pharmacy outpatient dispensing systems. Moreover, it is found in Zeng et al. ’s study (19) that the pharmacist is not the main constraint in discharge process delay, but rather, early release of discharge orders by physicians is the key to speeding up the discharge process.
For outpatient clinics, an orthopedic outpatient clinic is studied in Rohleder et al.’s study (20) to optimize staffing levels and patient scheduling. Werker et al. (21) describe the model to reduce planning time and waiting time in radiation therapy process. Berg et al. (22) shows that the maximum number of patients served in an endoscopy suite is linearly related to the number of procedure rooms, whose turnaround time has a significant impact on the utilization of procedure rooms and endoscopist. Patient scheduling is analyzed through simulations in Ogulata et al.’s study (23) to determine appropriate scheduling policy under different environmental conditions. Outpatient radiology scheduling procedure is analyzed in Lu et al.’s study (24) to reduce the number of tests without pre-approvals so that financial losses can be minimized. In addition, Villamizar et al. (25) analyzes the impacts of changes in patient volume, arrivals, and clinic scheduling. Reynolds et al. (26) studies the staffing model design for a health clinic for homeless people. A complete model of patient flow analysis in (27) shows that implementation of “swing” rooms (flexible between antepartum and mother-baby rooms) could help to balance bed allocation in a women’s health center. More DES models in various healthcare systems can be found in (28-34).
Discrete event simulation (DES) in lung cancer diagnosis process
To study lung cancer diagnosis process using DES, the simulation model can be constructed by following the paths in Figures 1-6. The following data are needed to define such a model.
Waiting time
This is the time between two consecutive steps or tests, i.e., the time a patient waits for the next test or diagnosis. Examples of the waiting time could be: the time between chest X-ray and CT scan in step 1&2; the time between CT-based biopsy and bronchoscopy in step 3; the time between step 1&2 and step 3, etc.
By checking the time stamps when the patients take each test, the waiting times for each patient can be collected. Then through statistical analysis, the collected waiting times are fitted into a distribution. The mean, the variance and other statistical parameters can be obtained. Such functions are included in most simulation software. These results are the time inputs to the simulation model.
Routing probabilities
The probability a patient may take one specific route or test. Examples of routing probabilities include: the probability a patient may only take CT scan in step 1&2; the probability a patient may take CT scan and brain imaging in step 4; the probability a patient will go to step 5 directly after step 3, etc.
By counting the number of patients in each possible route from one step, and dividing the total number of patients leaving that step, such probabilities can be calculated and will be the routing inputs to the simulation model.
Using the simulation model, a validation study can be carried out by comparing the simulation model output with the results obtained through data collection. If the difference is small enough, the simulation model is validated and can be used for further analysis, such as ‘what-if’ analysis. For example, by reducing one waiting time by 10%, we can evaluate its impact on the overall diagnosis time. By carrying out such activity for all the waiting times, one can compare the results and discover the activity leading to the largest reduction in overall diagnosis time. Such a waiting time is viewed as a ‘bottleneck waiting time’ or the ‘system constraint’. Then efforts can be focused on reducing the bottleneck waiting time. This effort can be repeated continuously until the overall diagnosis time reaches the desired value.
Analytical models
Markov chain model
To study the lung cancer diagnosis process, two types of analytical models could be useful. One is referred to as Markov chain, the other is closed formula.
Continuous time Markov chain (CTMC)
To briefly introduce the Markov chain model (35-37), consider a continuous time stochastic process X(t), t≥0, taking non-negative integer values. If for all s, t, u≥0, and non-negative integers i, j, k, the following property holds:
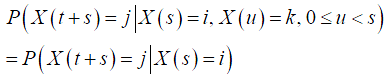
then such a process is a CTMC. In other words, in such a process, the conditional distribution of a future state at time t+s, given the current state at time t and all past states, only depends on the current state and is independent of the past states. Such a property is referred to as the Markovian property.
Introduce

to denote the probability that the process is in state j at time t+s, given that it is in state i at time s. Such a probability is referred to as the transition probability of the CTMC. If Pij(t) is independent of s, then the CTMC has stationary or homogeneous transition probabilities. When t→∞, the probability that a CTMC will be in state j often converges to a limiting value Pj, independent of the initial state, i.e.,
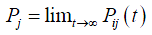
Such limiting probability exists if, given a process starts in state i, there exists a positive probability it is in state j and it takes a finite time returning to state i. Probability Pj represents the proportion of time the process is in state j.
For a CTMC, the amount of time the process stays in state i before transitioning to another state follows exponential distribution with rate νi. Then the transition rate that the process will transit from state i into state j is denoted as qij, i.e.,
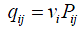
Then the rate that the process transits into state j equals to the rate that the process transits out of state j, i.e.,

where the left-hand side is the rate that the process leaves state j (flow-out), and the right-hand side is the rate that the process enters state j (flow-in). As one can see, such equations balance the flow-in and flow-out rates, so they are often referred to as balance equations. In addition, the sum of all the state probabilities equals to 1,
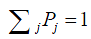
By solving these balance equations, Pj, the probability that the process is in state j can be obtained, which will lead to the performance measure of interest.
In addition to CTMC, discrete time Markov chain can be defined similarly. Consider a stochastic process X(n) at time n, n=1,2,. ., taking a finite or countable number of values and satisfying:
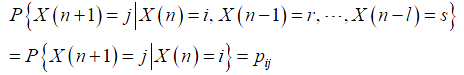
where X(n)=i implies that the process is in state i at time n. The transition probabilities and balance equations can be derived as well (35-37).
An illustrative example
To illustrate such a method, consider the two-service model introduced in (38), where a patient needs to go through nurse check and physician diagnosis within the patient room (or on patient bed). Denote the two services as s1 (nurse check) and s2 (physician diagnosis). Since both physician and nurse need to take care of multiple patients and they also have other duties in addition to meeting with patients, the status of their service is characterized by mi=1, i=1,2, if they are available and mi=0 otherwise. Let p1 represent the number of patients waiting for or being served by service s2. Since only one patient is allowed in the patient room, p1 could be either 0 or 1. Then, the states of the system are defined as {p1;m1,m2}. The probability the process stays in these states is denoted as P(p1;m1,m2).
Assume there is unlimited patient arrival. Each service has exponential service time with rate ci, and the providers have exponential available time and non-available time with rates λi and µi, respectively. From the Markov property that the rate of the system leaving a state should be equal to the rate of the system entering that state, the following balance equations are obtained (Box 1):

Full table
In addition, we have
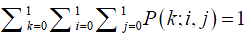
Solving these equations, all state probabilities P(k;i,j) can be obtained. According to Little’s law (39), flow time equals to number of patients divided by throughput. Since there is only one patient in the room, the patient length of stay Ts can be calculated as the inverse of throughput, i.e., the rate that the patient finishes physician diagnosis service.
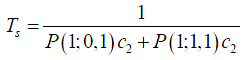
Markov chain model in healthcare systems
Markov chain model has been used extensively in many engineering and science fields, such as informatics, manufacturing, finance, medicine, physics and chemistry. In recent years, application of Markov chain in healthcare delivery systems has attracted a lot of research efforts.
As illustrated above, Wang et al. (38) models the care delivery activities inside a patient room in ED to evaluate patient length of stay and provider utilization. For general emergency medical service systems, Wiler et al. (6) reviews the available models for ED, including Markov chain and DES models. A two-dimensional Markov chain model is introduced in (40) to characterize the number of busy ambulances and whether the system is in compliance or not. The model can provide accurate estimates of response time distribution and number of busy ambulance distribution. Similarly, Almehdawe et al. (41) derives the steady state probability distributions of queue lengths and waiting times for ambulance patients. A three-hospital EMS-ED model is presented to analyze the impact of system resources on offload delays.
Patient flow and care deliveries have been studied using Markov chain models. A care activity model with multiple patient rooms and limited number of care providers in primary care clinics is presented in (42). Wang et al. (43) study work flow and staffing level in a CT test center and identify the imaging formatting process as the main constraint in the system. The patient flow in a gastroenterology clinic is evaluated in (44) based on a Markov chain model. Using this model, various policies on check-out scheduling are investigated. In addition, using a single room Markov chain model as a building block, an iterative method is introduced in (45) for a mammography imaging center with multiple rooms to study the work flow with a shared Technologist Assistant. In home care, Lanzarone et al. (46) introduces a Markov chain model of patient care pathway to provide predictions on number of patients who are followed up, the duration of each care and the amount of required visits, which can provide support for human resource planning.
Using the Markov chain model, hospital admissions have been studied. For example, Tang et al. (47) evaluate patient length of stay and use it to admit acute myocardial infarction patients into the hospital. It shows that the phase-type distribution can help account for the heavy skewness and heterogeneity in the data. The phase-type distribution is a convolution of exponential distributions, resulting from one or more inter-related Poisson processes occurring in sequence or phases. A survey of phase-type distribution modeling in healthcare systems is presented in (48) and ideas for further utilization are proposed (49); also studies hospital admission control and proposes a new gateway to improve admission through adding an expedited patient care queue. Using a Markov chain model of patient flow (50), discusses admission scheduling, resource requirement forecasting and resource allocation to satisfy demand and resource constraints.
Patient safety has been studied using Markov chain in (51), where the state space includes normal and risk status of patients, nurse check, physician intervention, and rapid response team (RRT) diagnosis. Through a recursive procedure, the limited availability of providers is considered when multiple patients are present. In addition, to improve patient safety in surgeries, the disruptions in surgical work flow are also modeled by Markov chain in (52), and bottleneck analysis is carried out to identify the most impeding disruption, removing which can reduce the impact of surgical disruptions in the strongest manner.
In addition, Markov chain has been used to model biologic processes, such as lung cancer growth and metastasis. In paper (53), the metastatic progression for primary lung cancer is modeled based on a Markov chain, which offers a probabilistic description of the time history of the disease unfolding through the metastasis cascade. This enables evaluation of disease progression pathways and timescales of progression from the lung to other sites. In (54), the progression of the disease is divided into four phases and calculated using a Markov chain model for familial nasopharyngeal carcinoma. Then four screening policies [(A) annual screening; (B) biennial screening; (C) triennial screening; and (D) triennial screening for participants who tested Epstein-Barr virus (EBV) negative and annual screening for participants who test EBV positive] are compared. The results show that screening policy (D) has the highest efficacy. Additional Markov chain models in health care applications can be found in (55-60).
Markov chain model of lung cancer diagnosis process
Using the CTMC outlined above, the lung cancer diagnosis process can be modeled. The system states can be defined as follows: Let the patient’s waiting for a test be a state of the process. For example, waiting for CT scan after chest X-ray in Figure 2, waiting for CT-based biopsy in Figure 3, and waiting for bone scan after brain imaging in Figure 4, can be defined as the states for the diagnosis process. Similarly, all other states can be defined.
From the collected data, the average waiting time can be calculated for each state. Reversing them we obtain parameter νi. The transition probability from one state to another one, Pij, will be the routing probability from one test to another. With these parameters, the balance equations can be obtained. Solving the equations, the overall diagnosis time is calculated.
Closed formulas
Due to the special feature in lung cancer diagnosis process, it is possible to develop closed formulas to evaluate the overall waiting time and the variability. It has been shown that for a serial process with multiple independent stages, the mean and variance of overall flow time will be equal to the sum of all process times and the associated variances, respectively. In other words, consider a serial process with M independent stages, if each stage i, i = 1,. .,M, takes an average time τi and variance vari to finish, then the mean T and variance Var of the overall diagnosis time will be:
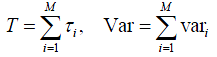
Such an approach has been used in studying the ‘Rapid Response’ process to improve patient safety in acute care. In papers (61-63), when deterioration in a patient’s clinical condition is detected, the primary nurse may call the intern, resident, or RRT for help. The provider can either make a decision or call for further help from the upper level physicians (e.g., intern to resident, RRT to resident, resident to fellow, fellow to attending). Thus, the response process can be modeled as a complex network with split, merge, and parallel structures. By considering the combination of possible routes (e.g., RN-intern-resident-fellow-attending), the closed formulas can be developed to evaluate the decision time and its variability.
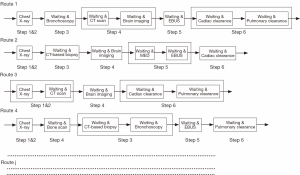
As shown in section 1, similar to the rapid response process, the lung cancer diagnosis process is very complicated and can also be modeled by a complex network. However, for one specific patient, he/she can only take one possible route. Thus, from his/her point of view, a serial process will be taken during the whole diagnosis period. Thus, by assuming all testing steps are independent, the closed formula can be applied for his/her route. To consider many patients, by including the routing probabilities, the whole diagnosis process can be represented by a combination of a set of specific routes, each being weighted through its routing probability. Figure 7 illustrates such an approach. The complex diagnosis process can be decomposed into a set of possible routes that the patients may go through. Four examples are illustrated in the figure. Then for a particular route j, the mean diagnosis time Tj and the variance Varj can be calculated. Calculating the product of all probabilities going to the next test after each one in this route, we obtain the probability αj of a patient taking such a route, which will be the weight of route j. Then αjTj and 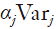 provide the weighted mean and variance, respectively. Summing them up, we obtain the final mean and variance of the overall diagnosis process.
provide the weighted mean and variance, respectively. Summing them up, we obtain the final mean and variance of the overall diagnosis process.
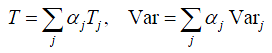
Discussion
Both the DES and analytical models are useful in studying the lung cancer diagnosis-to-treatment process. The simulation model can provide more detailed and more vivid analysis as well as user friendly graphic interface and animation. There are many DES software programs available, such as Simul8, Arena, Flexsim, ProModel (or MedModel). However, it takes longer time to develop and execute the simulation model, needs more inputs, and relies on the software environment. For complex processes and extensive scenarios, computation intensity may become an issue. More importantly, most simulation models are case-study based, which makes it difficult to discover some common features of the system.
The analytical models, on the other hand, can provide quick analysis, which is extremely useful during what-if analyses. In addition, it is possible to derive system properties, such as monotonically increasing property with respect to process parameters and bottlenecks. Also it requires less data inputs and is not dependent on software. However, the results are less detailed and do not have animation capability. The assumptions in the models may also limit their applications.
Concerning the analytical model for lung cancer diagnosis process, the Markov chain model assumes exponential service time, and may need a large number of states, which make the analysis difficult to proceed. Typically, empirical formulas need to be developed to approximate the performance in non-exponential scenarios (42-45). For small variations, i.e., the coefficient of variation (CV), defined as standard deviation divided by the mean, is small or less than 1, the average performance usually only depends on the mean and CV. In addition, it will be difficult to evaluate the variance. The closed formulas can handle any service time distributions and evaluate variance. However, the number of possible routes can be very big so that it may need to ignore some routes which have very small probabilities and have almost no impact on system performance. In both approaches, an independent assumption is introduced. In practice, a patient’s probability of receiving a certain test is usually conditioned on the previous test results. Thus the waiting time is also conditioned on the previous diagnostic results. Therefore, both the state dependent Markov chain and closed formula should be developed.
Conclusions
In this paper, computer process modeling methods are introduced for the lung cancer diagnosis-to-treatment process. Both DES and analytical models (including Markov chain model and closed formulas) can be used to estimate patients’ diagnosis-to-treatment time. Using these models, the complex relationship between waiting times and overall process time can be investigated, ‘what-if’ analyses can be carried out to determine the most critical waiting time that impedes early detection and staging in the strongest manner. Such methods provide quantitative tools and an alternative way to improve care quality in the lung cancer management process.
The methods introduced here are not only applicable to the lung cancer diagnosis process, but also useful in many healthcare delivery processes, such as patient or work flow, care transition, information transfer, as well as clinical decision process. The developed models can be used for staffing analysis, resource management, scheduling and decision support, among other things.
Acknowledgements
This work was supported by a grant from the Baptist Memorial Health Care Foundation (Dr. Li); and partially supported through a Patient-Centered Outcomes Research Institute (PCORI) Award (IH-1304-6147) to Dr. Osarogiagbon.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: RU Osarogiagbon, J Li.
Disclaimer: All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
References
- American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Jacobson SH, Hall SN, Swisher JR. Discrete-event simulation of health care systems. Patient Flow Reducing Delay in Healthcare Delivery 2006;91:211-52.
- Eldabi T, Paul RJ, Young T. Simulation modelling in healthcare: Reviewing legacies and investigating futures. J Oper Res Soc 2007;58:262-70.
- Gunal MM, Pidd M. Discrete event simulation for performance modelling in health care: A review of the literature. J Simulation 2010;4:42-51.
- Wiler JL, Griffey RT, Olsen T. Review of modeling approaches for emergency department patient flow and crowding research. Acad Emerg Med 2011;18:1371-9. [PubMed]
- Storrow AB, Zhou C, Gaddis G, et al. Decreasing lab turnaround time improves emergency department throughput and decreases emergency medical services diversion: A simulation model. Acad Emerg Med 2008;15:1130-5. [PubMed]
- Brenner S, Zeng Z, Liu Y, et al. Modeling and analysis of emergency department at University of Kentucky Chandler Hospital using simulations. J Emerg Nurs 2010;36:303-10. [PubMed]
- Zeng Z, Ma X, Hu Y, et al. Improving quality of care of emergency department at a community hospital: A simulation study. J Emerg Nurs 2012;38:322-8. [PubMed]
- Wang J, Li J, Tussey K. Reducing length of stay in emergency department: A simulation study at a community hospital. 1322 Ed. IEEE Transactions on Systems, Man, Cybernetics-Part A System and Human 2012;42:1314-22.
- Waleed AH, Amr A. Simulation-based framework to improve patient experience in an emergency department. Eur J Oper Res 2013;224:154-66.
- Konrad R, DeSotto K, Grocela A, et al. Modeling the impact of changing patient flow processes in an emergency department: Insights from a computer simulation study. Oper Res Health Care 2013;2:66-74.
- Griffiths JD, Price-Lloyd N, Smithies M, et al. Modelling the requirement for supplementary nurses in an intensive care unit. J Oper Res Soc 2005;56:126-33.
- Griffiths JD, Jones M, Read MS, et al. A simulation model of bed-occupancy in a critical care unit. J Simulation 2010;4:52-9.
- Zhu Z, Hen BH, Teow KL. Estimating ICU bed capacity using discrete event simulation. Int J Health Care Qual Assur 2012;25:134-44. [PubMed]
- Azari-Rad S, Yontef A, Aleman DM, et al. A simulation model for perioperative process improvement. Operations Research for health Care 2014;3:22-30.
- Lu T, Wang S, Li J, et al. Improving performance in the preparation and delivery of Antineoplastic medications at a community hospital: A simulation study. J Med Syst 2012;36:3083-9. [PubMed]
- Reynolds M, Vasilakis C, McLeod M, et al. Using discrete event simulation to design a mre efficient hospital pharmacy for outpatients. Health Care Manag Sci 2011;14:223-36. [PubMed]
- Zeng Z, Xie X, Zhong X, et al. Simulation modeling of hospital discharge process. Proceedings of Industrial and Systems Engineering Research Conference. San Juan, Puerto Rico, 2013:1383-90.
- Rohleder TR, Lewkonia P, Bischak D, et al. Using simulation modeling to improve patient flow at an outpatient orthopedic clinic. Health Care Manag Sci 2011;14:135-45. [PubMed]
- Werker G, Saur’e A, French J, et al. The use of discrete-event simulation modelling to improve radiation therapy planning processes. Radiother Oncol 2009;92:76-82. [PubMed]
- Berg B, Denton B, Nelson H, et al. A discrete event simulation model to evaluate operational performance of a colonoscopy suite. Med Decis Making 2010;30:380-7. [PubMed]
- Ogulata SN, Cetik MO, Koyuncu E, et al. A simulation approach for scheduling patients in the Department of Radiation Oncology. J Med Syst 2009;33:233-9. [PubMed]
- Lu L, Li J, Gisler P. Improving financial performance by modeling and analysis of radiology procedure scheduling at a large community hospital. J Med Syst 2011;35:299-307. [PubMed]
- Villamizar JR, Coelli FC, Pereira WCA, et al. Discrete event computer simulation methods in the optimisation of a physiotherapy clinic. Physiotherapy 2011;97:71-7. [PubMed]
- Reynolds J, Zeng Z, Li J, et al. Design and analysis of a health care clinic for homeless people using simulations. Int J Health Care Qual Assur 2010;23:607-20. [PubMed]
- Griffin J, Xia S, Peng S, et al. Improving patient flow in an obstetric unit. Health Care Manag Sci 2012;15:1-14. [PubMed]
- Cochran JK, Bharti A. Stochastic bed balancing of an obstetrics hospital. Health Care Manag Sci 2006;9:31-45. [PubMed]
- Coelli FC, Ferreira RB, Almeida RMVR, et al. Computer simulation and discrete-event models in the analysis of a mammography clinic patient flow. Comput Methods Programs Biomed 2007;87:201-7. [PubMed]
- Hung GR, Whitehouse SR, O’Neill C, et al. Computer modeling of patient flow in a pediatric emergency department using discrete event simulation. Pediatr Emerg Care 2007;23:5-10. [PubMed]
- Vasilakis C, Sobolev BG, Kuramoto L, et al. A simulation study of scheduling clinic appointments in surgical care: Individual surgeon versus pooled lists. J Oper Res Soc 2007;58:202-11.
- Ferreira RB, Coelli FC, Pereira WCA, et al. Optimizing patient flow in a large hospital surgical centre by means of discrete-event computer simulation models. J Eval Clin Pract 2008;14:1031-7. [PubMed]
- Paul JA, Lin L. Models for improving patient throughput and waiting at hospital emergency departments. J Emerg Med 2012;43:1119-26. [PubMed]
- Fanti MP, Mangini AM, Dotoli M, et al. A three level strategy for the design and performance evaluation of hospital departments. IEEE T Systems Man and Cybernetics Systems 2013;43:742-56.
- Ross SM, editor. Stochastic Process. New York: John Wiley & Sons, 1996.
- Ross SM, editor. Introduction to Probability Models, 11th ed. Oxford: Academic Press, 2014.
- Li J, Meerkov SM, editors. Production Systems Engineering. New York: Springer, 2009.
- Wang J, Li J, Howard PK. A system model of work flow in the patient room of hospital emergency department. Health Care Manag Sci 2013;16:341-51. [PubMed]
- Chhajed D, Lowe TJ, editors. Building intuition–Insights from basic operations management models and principles. International Series in Operations Research & Management Science. New York: Springer, 2008.
- Alanis R, Ingolfsson A, Kolfal B, editors. A Markov chain model for an EMS system with repositioning. Production and Operations Management. Year of Publication 2013;22:216-31.
- Almehdawe E, Jewkes B, He QM. A Markovian queueing model for ambulance offload delays. Eur J Oper Res 2013;226:602-14.
- Wang J, Zhong X, Li J, et al. Modeling and analysis of care delivery services within patient rooms: A system-theoretic approach. IEEE Transactions on Automation Science and Engineering 2014;11:379-93.
- Wang J, Quan S, Li J, et al. Modeling and analysis of work flow and staffing level in a computed tomography division of University of Wisconsin Medical Foundation. Health Care Manag Sci 2012;15:108-20. [PubMed]
- Zhong X, Song J, Li J, et al. Analysis and design of gastroenterology (GI) clinic in Digestive Health Center: A systems approach. Flex Serv Manuf J 2015. [Epub ahead of print].
- Zhong X, Li J, Ertl SM, et al. A systems theoretic approach for modeling and analysis of mammography testing process. IEEE Transactions on Systems, Man, and Cybernetics-Systems 2015. [Epub ahead of print].
- Lanzarone E, Matta A, Scaccabarozzi G. A patient stochastic model to support human resource planning in home care. Prod Plan Control 2010;21:3-25.
- Tang X, Luo Z, Gardiner JC. Modeling hospital length of stay by Coxian phase-type regression with heterogeneity. Stat Med 2012;31:1502-16. [PubMed]
- Fackrell M. Modelling healthcare systems with phase-type distributions. Health Care Manag Sci 2009;12:11-26. [PubMed]
- Helm JE, Beygi SA, van Oyen MP. Design and analysis of hospital admission control for operational effectiveness. Production and Operations Management 2012;20:359-74.
- Garg L, McClean S, Meenan B, et al. A non-homogeneous discrete time Markov model for admission scheduling and resource planning in a cost or capacity constrained healthcare system. Health Care Manag Sci 2010;13:155-69. [PubMed]
- Xie X, Li J, Swartz CH, et al. Modeling and analysis of ward patient rescue process on the hospital floor. IEEE Transactions on Automation Science and Engineering 2014;99:1-15.
- Shao X, Zhong X, Li J, et al. Bottleneck analysis to reduce surgical flow disruptions: Theory and application. IEEE Transactions on Automation Science and Engineering 2015;12:127-39.
- Newton PK, Mason J, Bethel K, et al. A stochastic Markov chain model to describe lung cancer growth and metastasis. PLoS One 2012;7:e34637. [PubMed]
- Choi CW, Lee MCH, Ng WT, et al. An analysis of the efficacy of serial screening for familial nasopharyngeal carcinoma based on Markov chain models. Fam Cancer 2011;10:133-9. [PubMed]
- Faddy MJ, McClean SI. Markov chain modelling for geriatric patient care. Methods Inf Med 2005;44:369-73. [PubMed]
- Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma ICU patients. J Trauma 2008;65:34-41. [PubMed]
- Zheng K, Padman R, Johnson MP, et al. An interface-driven analysis of user interactions with an electronic health records system. J Am Med Inform Assoc 2009;16:228-37. [PubMed]
- Eralp MN, Scholtes S, Martell G, et al. Screening of healthcare workers for tuberculosis: Development and validation of a new health economic model to inform practice. BMJ Open 2012;2:e000630. [PubMed]
- Rebuge A, Ferreira DR. Business process analysis in healthcare environments: A methodology based on process mining. Inf Syst 2012;37:99-116.
- Jeong S, Youn CH, Kim YW. Predicted cost model for integrated healthcare systems using Markov process. Ubiquitous Information Technologies and Applications Lecture Notes in Electrical Engineering 2014;280:181-7.
- Xie X, Li J, Swartz CH, et al. Modeling and analysis of rapid response process to improve patient safety in acute care. IEEE Transactions on Automation Science and Engineering 2012;9:215-25.
- Xie X, Li J, Swartz CH, et al. Improving response-time performance in acute care delivery: A systems approach. IEEE Transactions on Automation Science and Engineering 2015;11:1240-9.
- Xie X, Li J, Swartz CH, et al. Analysis of multi-patient rapid response processes: An iterative approach. Proceedings of IEEE International Conference on Automation Science and Engineering. Gothenburg, Sweden, 2015.