Erlotinib therapy after initial platinum doublet therapy in patients with EGFR wild type non-small cell lung cancer: results of a combined patient-level analysis of the NCIC CTG BR.21 and SATURN trials
Introduction
The aggregate 5-year survival rate of patients with non-small cell lung cancer (NSCLC) is 16%. Almost half of all patients present at diagnosis with stage IV disease, which has a 5-year survival rate of 4% (1). Majority of patients who present with earlier stage disease will die with relapsed or metastatic disease, even after curative-intent treatment (2). Although usually incurable, stage IV NSCLC can be treated with the expectation of a modest, but significant, improvement in life expectancy and quality of life. Unfortunately, all patients with advanced NSCLC will develop progressive disease at some point even after effective first line systemic therapy.
The median progression-free survival (PFS) after second line therapy for NSCLC is about 2-3 months (3,4). The landmark clinical trial National Cancer Institute of Canada Clinical Trials Group BR.21 (BR.21), demonstrated the superiority of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) erlotinib over placebo in controlling disease, delaying progression and prolonging survival in patients whose disease had progressed after one or two prior lines of chemotherapy (5). Erlotinib also delayed the onset of disease progression and prolonged survival in the Sequential Tarceva in Unresectable Non-Small Cell Lung Cancer (SATURN) trial, a placebo-controlled trial of a modified strategy in which patients who had responded to a pre-determined number of platinum doublet chemotherapy cycles were immediately started, and continued until treatment failure, on second-line (‘switch maintenance’) therapy prior to evident failure of first line therapy (6).
The striking response of patients with activating mutations of EGFR to TKI therapy has raised the possibility that the benefit of erlotinib in the switch maintenance and post-first-line therapy setting may be predominantly attributable to benefit in subsets of patients with activating mutations of EGFR. This notion is supported by data in the frontline setting, where the use of a different EGFR TKI in patients with wildtype EGFR was associated with worse outcomes than the use of conventional chemotherapy (7). Because activating mutations of EGFR are present in only 10-16% of NSCLC patients in Western populations absence of benefit in wild-type patients would imply that the use of EGFR TKI therapy is futile in the majority of patients (8,9). Unfortunately, because of the timing of discovery, neither BR.21 nor SATURN prospectively tested the relationship between mutation status and treatment effects, leaving open the possibility that the observed benefit is limited to patients with activating mutations of EGFR.
However, the EGFR pathway is activated in NSCLC with wild-type EGFR lacking the excessive kinase activity of the mutant EGFR receptor, which accounts for the oncogenic effect of driver mutations of EGFR (8,10). This more modest activation of the EGFR pathway is nevertheless cancer-promoting by stimulating cell proliferation, inhibiting apoptosis, stimulating angiogenesis, cellular motility and invasiveness (10). Therefore, we hypothesized that erlotinib therapy benefits patients with EGFR wild type NSCLC after prior platinum doublet chemotherapy. We examined the subset of patients in BR.21 and SATURN who were documented to have wildtype EGFR. Our objective was to quantify the survival impact of erlotinib therapy in these patients.
Patients and methods
Sources of data
We obtained patient-level information from BR.21 and SATURN, which are both large double-blind prospective randomized, placebo-controlled clinical trials of erlotinib therapy in patients who had already received conventional platinum-doublet chemotherapy. Majority of patients enrolled in BR.21 were from North and South America. Patients with Eastern Cooperative Oncology Group (ECOG) performance status 0 to 3, who had progressed after first or second line therapy (including a platinum doublet), were eligible. Key eligibility criteria and other details of this study have been published (5). In the SATURN trial, patients with ECOG performance status 0-1 who had stable or responsive disease after up to 4 cycles of platinum doublet chemotherapy were randomized to early second line therapy (now referred to as switch maintenance therapy) with either erlotinib or placebo. The SATURN trial enrolled patients from multiple sites around the world, but predominantly from Europe (6).
Algorithm for mutation testing
The predictive and prognostic value of EGFR mutations was first reported in 2004, well after enrollment of patients into BR.21, but during enrollment for SATURN (11-13). There was no mandate for submission of tissue with enrollment into BR.21 and all available information on the mutation status of patients on this study has been obtained retrospectively (14). Tissue submission was mandated in the SATURN trial, however, the hierarchy of testing placed greater emphasis on EGFR expression testing by immunohistochemistry (IHC) and fluorescent in-situ hybridization (FISH) in that order, with testing for EGFR mutation third in priority and only performed when sufficient tissue remained (15). Consequently, only 25% of the BR.21, and 49% of the SATURN population had their EGFR mutation status available.
Statistical analyses
We compared PFS and overall survival (OS) outcomes between the erlotinib and placebo treatment groups within the EGFR wild-type subpopulation according to the intention-to-treat principle. PFS was measured from the date of randomization until investigator-assessed disease progression or death from any cause. OS was measured from the date of randomization until death from any cause. The PFS and OS distributions were estimated by Kaplan-Meier curves and compared using the two-sided log-rank test. The Cox proportional hazards regression model was used to estimate hazard ratios (HR) (erlotinib vs. placebo) adjusted for potential baseline confounders (ECOG performance status, age, race, sex, smoking history, histology, and duration of time from initial diagnosis of NSCLC to study randomization). Analyses of combined data further stratified by study (SATURN vs. BR.21) when comparing erlotinib vs. placebo.
We analyzed the BR.21 and SATURN studies separately, and also performed a pooled analysis, in which we combined patient-level data across the EGFR wild-type BR.21 and SATURN study populations. We performed additional analyses to evaluate the comparability of baseline characteristics and survival outcomes between the patients with known and unknown EGFR status information in order to assess the generalizability of the BR.21 (25% known) and SATURN (49% known) EGFR wild type study subpopulations. Because post-study therapy was initiated at the discretion of the investigator, we also performed additional analyses using a Cox proportional hazards model to adjust for the impact of post-study therapies on OS comparisons.
Results
In BR.21, 184 of 731 (25%) enrolled patients had their mutation status determined; in SATURN, 437 of 889 (49%) were determined. The key clinical and demographic characteristics of patients were similar between erlotinib and placebo treated patients known to have wild-type EGFR in both trials (Table 1). In the BR.21 study, which used a 2:1 randomization schema, 102 of 117 (87%) patients randomized into the erlotinib treatment arm and 48 of 67 (72%) patients randomized into the placebo arm are known to have wild-type EGFR. The median PFS was 2.2 months for patients treated with erlotinib, compared to 1.8 months in patients who received placebo [HR 0.55; 95% CI, 0.37-0.81; P<0.005 (Figure 1A)]. The median OS was 8.1 months with erlotinib therapy, compared to 3.4 months with placebo therapy [HR 0.68; 95% CI, 0.45-1.02; P=0.144 (Figure 1B)].
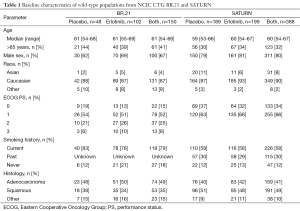
Full table
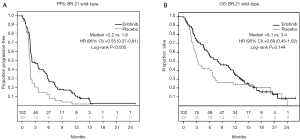
In the SATURN study, 199 of 221 (90%) patients randomized to treatment with erlotinib and 189 of 216 (88%) randomized to placebo therapy had wild-type EGFR. The median PFS was 2.8 months with erlotinib therapy and 2.0 months with placebo [HR 0.76; 95% CI, 0.62-0.94; P=0.018 (Figure 2A); the median OS was 11.3 months from the time of randomization for patients who were assigned to the erlotinib maintenance arm, vs. 10.2 months in the placebo maintenance cohort (HR 0.72; 95% CI, 0.57-0.91; P=0.024 (Figure 2B)]. The SATURN study evaluates survival over a longer period in part because patients were enrolled into the trial shortly after completing 4 cycles of first line platinum doublet therapy, before they had any evidence of disease progression.
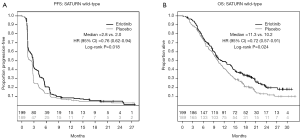
The comparison of PFS, when patients on both trials were combined, revealed a significant improvement in patients treated with erlotinib, compared to those who received placebo [HR 0.71; 95% CI, 0.59-0.85; P<0.01 (Figure 3A,B)]. Analysis of key clinical and demographic subgroups in the combined population reveals that the benefit of erlotinib was maintained in all groups of patients (Figure 3C). The OS comparison in the combined study population similarly revealed a significant benefit in recipients of erlotinib therapy [HR 0.72; 95% CI, 0.59-0.88; P<0.01 (Figure 4)]. Sensitivity analyses of OS (in the combined trial population) which adjust for post-study therapy also suggested a benefit of erlotinib therapy compared to placebo (HR 0.75; 95% CI, 0.61-0.92; P=0.005).
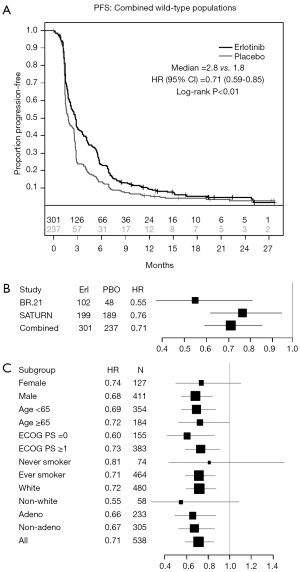
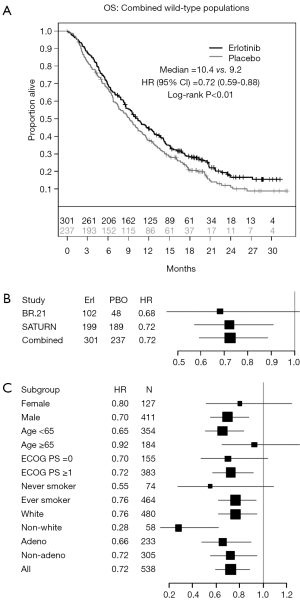
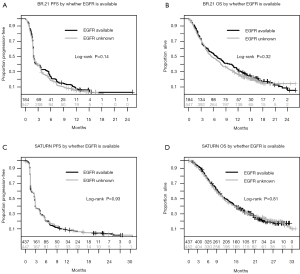
Figure 5 displays the Kaplan-Meier curves comparing PFS and OS distributions between patients whose EGFR status was known vs. unknown in the SATURN and BR.21 studies. The log-rank P values for each comparison are all >0.14 suggesting that the PFS and OS distributions do not differ according to whether EGFR status was known. In addition, baseline characteristics were generally well balanced between known and unknown EGFR status in both studies (data not shown). These results suggest that while the EGFR status was known for only a subset of the ‘intention-to-treat’ population (25% for BR.21 and 49% for SATURN), analyses based on the study population with known EGFR status may be representative of the overall study population.
Discussion
Optimizing survival of patients with metastatic NSCLC entails effective use of currently available systemic therapy. Virtually all recipients of first line chemotherapy for stage IV NSCLC will experience disease progression at some point. At that time, the therapeutic options for further extension of life are limited to a few drugs. Erlotinib is one of the few drugs currently approved by regulatory agencies, such as the US Food and Drug Administration and the European Medicines Agency, for use in this setting. Optimization strategies have gone from conventional line-of treatment-directed therapy, to a switch maintenance strategy which is essentially a strategy of early second line therapy with the putative advantage of guaranteeing exposure to effective drugs before clinical events, such as worsening performance status, preclude the possibility of treatment (6,16-18).
The dramatic responses and remarkable improvement in PFS seen in patients with activating mutations of EGFR naturally raise questions about whether the survival benefits found in unselected populations might have been due primarily to the benefit in the minority population with these mutations (19,20). We need to know if EGFR TKIs do not provide demonstrable benefit in patients with EGFR wild-type NSCLC, in order to avoid exposing such patients to futile therapy. The temporal evolution of knowledge about EGFR mutations came too late for prioritization of prospective mutation testing in the two landmark clinical trials of erlotinib therapy. We have therefore performed a combination analysis using patient level data to quantify the benefit of erlotinib therapy in the EGFR wildtype population.
We found a consistent and clinically meaningful risk reduction of approximately 30% in patients with wildtype EGFR who were treated with erlotinib in the individual studies, and in the combination of studies. Our analysis suggests that patients with wildtype EGFR who have disease control after initial platinum doublet chemotherapy (SATURN), and those with disease progression after first line platinum doublet chemotherapy (BR.21), benefit from erlotinib therapy (Figure 4C). However, the unplanned, retrospective nature of this analysis raises the possibility of imbalance in key prognostic factors between the groups. For example, only a minority of patients enrolled into both studies are known to be EGFR wild-type because a large proportion of patients were never tested. Although our additional analyses suggest that the results in the subset we have analyzed can be generalized to the whole enrolled population (Figure 5), we cannot directly confirm this.
Other potential sources of bias in this study include the heterogeneity of the study populations. For example, BR.21 included patients undergoing salvage second- and third-line systemic therapy for relapsed disease and expanded eligibility to patients with relatively poor performance status and the results therefore reflect the predominance of patients with poorer prognosis than those enrolled into SATURN. However, we felt justified in combining these two studies because they were both double-blind and placebo controlled, enabling us to test the primary question of efficacy of erlotinib. Although we performed statistical modeling to control for the impact of subsequent therapy on the OS analysis, this is still theoretically a source of bias.
In the SATURN trial, patients randomized to the erlotinib arm technically received salvage third-line therapy at the time of progression, compared to second-line therapy in the placebo arm and whereas all patients on the erlotinib arm were exposed to potentially effective second-line therapy (erlotinib), 34% of the patients randomized to placebo never received second line therapy at the time of progression (6). Because of the technical structure of placebo-controlled switch maintenance therapy trials, post-progression therapy was actually third-line therapy for the 60% of patients on the erlotinib arm who went on to post-study therapy upon progression, vs. second-line therapy for the 66% of patients randomized to placebo who went on to post-study therapy. However, the consistency between the PFS and OS data in our analysis suggests a significant independent benefit from erlotinib therapy.
The relevance of our analysis to contemporary practice may be questioned. After all, in today’s practice, no one would offer placebo therapy to patients after first line systemic therapy for stage IV NSCLC. Therefore, a more relevant question might be the comparative efficacy of erlotinib or conventional salvage chemotherapy in this setting. Although multiple studies have shown equivalence in all end-points of interest including response rates and survival when second line therapy with EGFR TKI’s has been compared to chemotherapy, these studies have included an unselected population (21,22). Therefore the question of comparative efficacy in patients with EGFR wild-type NSCLC has been open to debate. One attempt to address the comparative effectiveness question, the TAILOR trial, suggested that Docetaxel therapy was associated with superior disease control, and PFS, and OS in comparison to erlotinib in the second line therapy setting (23,24). Unfortunately, this open-labeled study had major methodologic problems, which have left the question open to debate (25-27). Furthermore, it is worthwhile to point out that up to 40% of patients who receive first line systemic chemotherapy for NSCLC never proceed to second-line therapy, even in clinical trials (6,16-18). Poor understanding of the state of the evidence might lead clinicians to believe that erlotinib has no benefit in patients with wildtype EGFR. This report clearly points out the error of that belief. Until curative therapy for stage IV lung cancer becomes available, quantifying the incremental value of relatively well tolerated therapy over placebo will remain relevant in contemporary practice.
A more interesting challenge is to distinguish subsets of EGFR wildtype NSCLC patients who do, and do not, benefit from EGFR TKI therapy. Several investigators have tackled this problem with some success, including the use of proteomic signatures to predict the probability that EGFR wildtype patients would, or would not, respond to erlotinib therapy. One such study retrospectively applied a proteomic signature to the BR.21 wildtype subset (28). The use of this test was subsequently validated in a clinical trial (25,29). EGFR copy number, protein expression, and micro-RNA 120b expression have also been reported to help predict NSCLC response to TKI therapy (30-33). We are unable to address this question in the current data analysis.
In summary, we have demonstrated that the benefit of erlotinib therapy in BR.21 and SATURN was not limited to patients with activating mutations of EGFR. Significant clinical benefit was achieved in patients with EGFR wild-type NSCLC. Therefore erlotinib remains a viable option for treating EGFR wildtype patients with NSCLC after prior frontline systemic chemotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Osarogiagbon: speaker, consultant Roche/Genentech; consultant, Eli Lilly. Dr. Cappuzzo: none. Dr. Ciuleanu: consultant, Roche; honoraria, Roche. Dr. Leon: employment, Genentech/Roche; stock ownership, Genentech/Roche. Dr. Klughammer: employment and stock ownership, Hoffman-LaRoche. This manuscript has been presented at the American Society of Clinical Oncology Meeting, Chicago, IL, June 2013.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [PubMed]
- Herbst RS, Bunn PA Jr. Targeting the epidermal growth factor receptor in non-small cell lung cancer. Clin Cancer Res 2003;9:5813-24. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [PubMed]
- Zhu CQ, da Cunha Santos G, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008;26:4268-75. [PubMed]
- Brugger W, Triller N, Blasinska-Morawiec M, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:4113-20. [PubMed]
- Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:591-8. [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [PubMed]
- Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [PubMed]
- Lee DH, Park K, Kim JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res 2010;16:1307-14. [PubMed]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. [PubMed]
- Jassem J, Dziadziuszko R. EGFR inhibitors for wild-type EGFR NSCLC: to use or not to use? Lancet Oncol 2013;14:916-7. [PubMed]
- Butts CA. VeriStrat validated in patients with non-small-cell lung cancer. Lancet Oncol 2014;15:671-2. [PubMed]
- Moro-Sibilot D, Perol D, Chabaud S, et al. The TAILOR study: to agree or to disagree? Lung Cancer 2014;84:315-6. [PubMed]
- Ruiz J, Petty WJ. Clinical perspective on PROSE: does VeriStrat testing improve selection of second-line treatment for patients with non-small cell lung cancer? Ann Transl Med 2015;3:31. [PubMed]
- Carbone DP, Ding K, Roder H, et al. Prognostic and predictive role of the VeriStrat plasma test in patients with advanced non-small-cell lung cancer treated with erlotinib or placebo in the NCIC Clinical Trials Group BR.21 trial. J Thorac Oncol 2012;7:1653-60. [PubMed]
- Gregorc V, Novello S, Lazzari C, et al. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol 2014;15:713-21. [PubMed]
- Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst 2005;97:643-55. [PubMed]
- Hirsch FR, Varella-Garcia M, Cappuzzo F, et al. Combination of EGFR gene copy number and protein expression predicts outcome for advanced non-small-cell lung cancer patients treated with gefitinib. Ann Oncol 2007;18:752-60. [PubMed]
- Hirsch FR, Varella-Garcia M, Dziadziuszko R, et al. Fluorescence in situ hybridization subgroup analysis of TRIBUTE, a phase III trial of erlotinib plus carboplatin and paclitaxel in non-small cell lung cnacer. Clin Cancer Res 2008;14:6317-23. [PubMed]
- Weiss GJ, Bemis LT, Nakajima E, et al. EGFR regulation by microRNA in lung cancer: correlation with clinical response and survival to gefitinib and EGFR expression in cell lines. Ann Oncol 2008;19:1053-9. [PubMed]


