Editorial preface to this special issue “Improving the quality and outcomes of lung cancer care: an interdisciplinary approach”
The challenge of quality improvement—defining quality, identifying gaps, implementing effective and sustainable change
King: Once more unto the breach, dear friends, once more
Or close the wall up with our English dead—(1)
‘For as the body without the spirit is dead, so faith without works is dead also’ (2).
There are many dead things in life. Faith without works is dead. So is knowledge without implementation. Lung cancer is the oncologic public health challenge of our age. The statistics are Homeric: 1.6 million men and women diagnosed worldwide annually; 1.4 million dead annually (3). Despite knowledge of the enemy since the 1950s, there has been little progress made until recently in combating the grip of tobacco on society. But, these are exciting times in thoracic oncology. There is growing public will to tackle the challenge of tobacco control, technologic advances have finally provided an avenue for early detection, and less morbid approaches to treatment across the full spectrum of disease stage.
Challenges remain. Fifty-one years after the US Surgeon General’s initial report directly linking tobacco with lung cancer, the tobacco industry has managed to thrive in the US and has establish major beachheads in some of the most populous countries on earth, such as China and India (4). CT screening, with all its promise, holds real dangers when applied in the real-world communities where the real opportunity predominates (5); fundamental issues of healthcare and outcome disparities flourish across the board (6). Simply put, there is a major gap between knowledge and implementation across the full spectrum of lung cancer care from tobacco control to palliative care. This quality gap is a major breach into which our lung cancer dead will continue to be buried. As with Shakespeare’s young English king’s desperate exhortation to his flagging troops, we know that all our lung cancer dead will not close up this peculiar wall. Only the living can. Closing this wall is the particular challenge of implementation science.
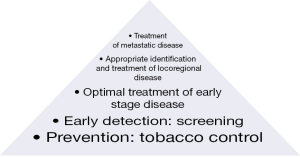
Logically examined, the opportunities to eliminate the lung cancer epidemic are greatest with tobacco control, next greatest with effective screening, and so on, sequentially, out to the treatment of advanced disease and end-of-life care (Figure 1). Currently our clinical and research resources are spent in almost exactly the opposite direction. In this special issue of Translational Lung Cancer Research (TLCR), we seek to engender dialogue on improving lung cancer survival at the broad population level. Our team of contributors spans the globe and spans the spectrum of perspectives on lung cancer care delivery from academic researchers to community-level care providers. The topics span the spectrum of lung cancer care from epidemiology and tobacco control, to diagnosis, treatment and outcomes improvement. We have sought to provide a forum for an evidence-based discussion of the challenges of delivering high quality care to patients with lung cancer, in the places where they choose to seek care; quantify the breach in the quality wall; examine scientifically rigorous and practical steps to close the breach and thereby reduce the numbers of our lung cancer dead.
Dr. Jemal and his team at the American Cancer Society provide an overview of the geographic and demographic epidemiology of lung cancer, including variations in quality and outcomes of care from a worldwide perspective (3). They provide an overview of the incidence, mortality rates and trends of lung cancer, discuss current knowledge about the mortality burden and differences between populations within and across geographic boundaries. By sizing up the enormity of the challenge that is lung cancer, they set the stage for the quality and outcomes improvement discussions to come in subsequent papers.
Farjah and Detterbeck in their paper titled ‘What is quality, and can we define it in lung cancer care?’ discuss the need for Quality Improvement in lung cancer care, as a strategy for improving the survival of large populations of lung cancer patients (7). They discuss the challenge in developing evidence-based markers of quality from diagnosis, through staging, to treatment, and the potential impact on patient outcomes. They provide a conceptual framework for defining and identifying ‘quality care’, and summarize current and potential future trends in the lung cancer quality improvement movement.
Yu and colleagues from the University of Memphis School of Public Health discuss the challenges and opportunities posed by the effort to measure quality improvement in populations, from the implementation science perspective (8). They present methodologic aspects of change, including frameworks for measuring change and its impact in populations; alternative study designs, including randomization and non-randomization strategies for intervention studies. Memphis, in the US state of Tennessee, is at the heart of the lung cancer kill-zone of America. Their perspective is therefore informed by battle field experience.
The tumor, node and metastasis system is our most important means of communicating all things about lung cancer. Therefore, lung cancer quality improvement must communicate in this language. Drs Rami-Porta, Asamura, and Goldstraw, current chair, chair-elect, and immediate past-chair, respectively, of the International Association for the Study of Lung Cancer (IASLC) Staging and Prognostic Factors Committee discuss this language and its evolution in our ‘molecular age’ (9).
Lathan in his paper titled ‘Lung cancer care: the impact of facilities and area measures’, discusses the arena of care and its impact on patient outcomes, giving a brief overview of current research on institutional and other non-clinical determinants of lung cancer patient outcomes (6). This discussion mostly avoids the somewhat hackneyed Jeremiad about patient-level disparities in care delivery and outcomes, and provides an early glimpse of pathways into corrective interventions at the provider and institutional levels.
Freeman, Krasna, and this guest editor discuss models of the arena of care, with emphasis on the much-touted multidisciplinary care model (10). In our paper titled ‘Implementing effective and sustainable multidisciplinary thoracic oncology programs’, we break lung cancer care delivery into three conceptual models, discuss the pros and cons of each, and the evidence to support them. We also emphasize the need for a stakeholder-centric approach, in which key stakeholders in lung cancer care are carefully identified, and their perspectives on care delivery goals clarified and reconciled in order to develop meaningful benchmarks with which to develop and measure care delivery programs. All three contributors to this paper are active lung cancer clinicians, with hybrid academic and community-based experience in lung cancer care, who have executed multidisciplinary thoracic oncology programs within non-University-based clinical care environments.
After these six ‘infrastructural’ papers, we dig into specific components of the lung cancer care delivery challenge, starting with Warren and Ward’s paper titled ‘Integration of tobacco cessation services into multidisciplinary lung cancer care: rationale, state of the art, and future directions’, as self-explanatory a title as one will ever get (11)! They discuss the value and impact of smoking cessation after diagnosis of lung cancer, and make recommendations for smoking cessation program implementation within the arena of care delivery, from lung cancer screening to management of advanced lung cancer patients.
Speaking of screening, Chiles and Optican cover the history of lung cancer screening up to the resounding success of the National Lung Screening Trial (NLST), and down to the inevitable ‘so it works, now what?’ question. In their paper titled ‘Implementing lung cancer screening in the real world: opportunity, challenges and solutions’, they discuss the current evidence in support of CT screening for lung cancer (5). They provide a brief history of failed prior attempts at screening, summarize the data from the NLST (including cost-effectiveness analyses), issues for consideration in implementing screening programs at community-level institutions, benefits of screening, the need for (and types of) data collection with implementation of screening programs, the potential impact of screening on lung cancer incidence, stage distribution and mortality. They also discuss the importance of the environment of care in implementing lung cancer screening programs; the potential hazards of screening; current and future challenges and opportunities. We must heed the lessons of cervical, breast and colorectal cancer screening, as we seek to use this incredible breakthrough to save the lives of high risk patients in our communities.
Dr. Folch and colleagues, in their paper ‘Lung cancer diagnosis and staging in the minimally invasive age with increasing demands for tissue analysis’, tackle a very trenchant problem in lung cancer care delivery (12). As the countervailing forces of our ravenous hunger for tissue in this molecular age of lung cancer collides with technological advances promoting minimally-invasive approaches to lung cancer diagnosis and staging, they provide a state-of-the art discussion of tissue procurement for diagnosis, staging, and treatment selection. They discuss the range of options, their performance characteristics, advantages and disadvantages; and provide an evidence-based recommendation for strategic use of tissue procurement options, such as combinations of endoscopic ultrasound techniques with mediastinoscopy; the comparative economics of such complementary testing; and the feasibility of using such techniques in different environments of care, ranging from comprehensive academic/research institutions to community-level institutions.
Acknowledging that lung cancer care is only as good as pathology examination, we have a comprehensive, evidence-based discussion of challenges in ‘Improving the pathologic evaluation of lung cancer resection specimens’, by a team of community pathologists in a region of the US with some of the highest lung cancer incidence and mortality rates (13). This group, the Mid-South Pathology Quality Improvement Consortium is currently engaged in a regional dissemination and implementation project to improve the quality of pathologic examination of lung resection specimens in 14 community level hospitals in five Dartmouth Hospital Referral Regions in three states, Mississippi, Arkansas and Tennessee, states with the 2nd, 3rd, and 4th highest lung cancer mortality rates in the US. In this paper, we discuss current understanding of optimal pathologic examination of lung cancer specimens, novel methods of pathology examination of lung cancer specimens, define the quality gap in pathology, its impact on patient outcomes, challenges pathologists face in handling lung cancer specimens, modern responses to these challenges, such as synoptic reporting, and interventions to improve lymph node yield for more accurate pathologic nodal staging.
D’Amico and I discuss the challenge of improving lung cancer outcomes by improving the quality of surgical care, recognizing that surgery is the key curative modality for lung cancer now and into the foreseeable future (14). We discuss the quality gap in surgical management of lung cancer, its implications for patient survival, and means to measure surgical quality as the first step to eliminating the quality gap. Dr. D’Amico also provides a state-of-the-art discussion of the evidence for minimally invasive resection techniques, emphasizing the maintenance of oncologic quality of resection. This paper ends by looking through a glass darkly, trying to forecast the role of surgery in a world with increasingly competitive non-surgical options for curative-intention care.
With advances in technology and the advent of CT-screening, the issue of non-surgical treatment of patients with early stage lung cancer has become a casus belli between surgeons, radiation oncologists, and interventional radiologists. In their paper, ‘Triaging early stage patients into non-surgical pathways’, Drs. Sroufe and Kong (one a community, the other an academic, radiation oncologist) discuss the evidence for stereotactic body radiation, radiofrequency ablation, and other non-surgical means of definitive local treatment of early stage lung cancer, and the direction in which things might be heading (15).
In one of the two original contributions in this special issue, we present a somewhat counter-intuitive conundrum in this age of targeted therapy for lung cancer: evidence that the tyrosine kinase inhibitor, Erlotinib, has demonstrable beneficial effects in patients with stage IV epidermal growth factor receptor wildtype lung cancer (16). What to do? This inconvenient truth will not go away…
The second original contribution is a qualitative evaluation of two of the most important stakeholders in lung cancer care- patients and their home caregivers- and their subjective feelings about models of care delivery for lung cancer (17). This method of research, focus groups of a research population in which seemingly free-flowing conversations are recorded and analyzed using a rigorous method that looks for themes in conversation and attempts to parse meaning by plumbing the depths of human perceptions, is one that might seem alien to most lung cancer researchers. But this is a whole world that exists with tools germane to our eternal quest to serve our patients better by seeing things through their lived experience.
We end this special issue with a paper written by a team from the College of Engineering at the University of Wisconsin, Madison, in conjunction with the Thoracic Oncology Research team at a community-level health care system, which seems the diametric opposite of the preceding paper. ‘Computer modeling of lung cancer diagnosis-to-treatment process’ is an uber-quantitative example of process engineering in which mathematical simulation models are used to examine the process of care delivery, in order to identify the locations and causes of bottlenecks within a healthcare system (18). I ask readers not to be intimidated by the proliferation of mathematical symbols in this paper. In many ways, to quote the philosopher Marshall McLuhan, ‘the medium is the message’ in this paper.
In summary, the landscape of lung cancer changes apace, with an accelerating rate of discovery in the realm of cancer biology, the advent of CT scan screening, improvements in our technical ability to do more with less invasiveness. Along with this expansion of beneficial knowledge and intervention options, has come an exponential expansion of the complexity of decision-making and technical skillsets required to deliver optimal care. Faith without works is dead. So is discovery without implementation. Dead.
We can use rigorous scientific methods to improve the quality of care delivery for lung cancer. We just have to decide what ‘quality of care’ looks like, so we can identify it when we see it, and know when we don’t, perchance answer the question ‘why is it missing’, and thereby find it and bring it home where it belongs. In the end, the societal value of progress from discovery in lung cancer must be measured at the broad population level. It will not count for much if we do not bend down the incidence and mortality curves for lung cancer.

Acknowledgements
I want to thank all the contributors to the various papers in this special issue for their tremendous effort. Also to the editorial staff at TLCR for their grace under pressure.
Funding: Supported by RO1 CA172253 and PCORI IH-1304-6147. This work was (partially) supported through a Patient-Centered Outcomes Research Institute (PCORI) Award (IH-1304-6147).
Footnote
Conflicts of Interest: The author has declared no conflicts of interest.
Disclaimer: All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
References
- William Shakespeare. Henry V: Act 3, scene 1, lines 1-2. In: TW Craik, editor. King Henry V; The Arden Shakespeare 3rd series: Proudfoot R, Thompson A, Kastan DS, et al, editors. London: Thompson Learning, England, 2011.
- The Official King James Bible Online. James 2:26. King James Version. Available online: http://www.kingjamesbibleonline.org/James-2-26/
- Islami F, Torre LA, Jemal A. Global trends in lung cancer mortality and smoking prevalence. Transl Lung Cancer Res 2015;4:327-38.
- The Reports of the Surgeon General: The 1964 Report on Smoking and Health. Available online: http://profiles.nlm.nih.gov/ps/retrieve/Narrative/NN/p-nid/60, accessed on Aug 7, 2015.
- Optican RJ, Chiles C. Implementing lung cancer screening in the real world: opportunity, challenges and solutions. Transl Lung Cancer Res 2015;4:353-64.
- Lathan CS. Lung cancer care: the impact of facilities and area measures. Transl Lung Cancer Res 2015;4:385-91.
- Farjah F, Detterbeck FC. What is quality, and can we define it in lung cancer?—the case for quality improvement. Transl Lung Cancer Res 2015;4:365-72.
- Yu X, Klesges LM, Smeltzer MP, et al. Measuring improvement in populations: implementing and evaluating successful change in lung cancer care. Transl Lung Cancer Res 2015;4:373-84.
- Rami-Porta R, Asamura H, Goldstraw P. Predicting the prognosis of lung cancer: the evolution of tumor, node and metastasis in the molecular age—challenges and opportunities. Transl Lung Cancer Res 2015;4:415-23.
- Osarogiagbon RU, Freeman RK, Krasna MJ. Implementing effective and sustainable multidisciplinary clinical thoracic oncology programs. Transl Lung Cancer Res 2015;4:448-55.
- Warren GW, Ward KD. Integration of tobacco cessation services into multidisciplinary lung cancer care: rationale, state of the art, and future directions. Transl Lung Cancer Res 2015;4:339-52.
- Folch E, Costa DB, Wright J, et al. Lung cancer diagnosis and staging in the minimally invasive age with increasing demands for tissue analysis. Transl Lung Cancer Res 2015;4:392-403.
- Osarogiagbon RU, Hilsenbeck HL, Sales EW, et al. Improving the pathologic evaluation of lung cancer resection specimens. Transl Lung Cancer Res 2015;4:432-7.
- Osarogiagbon RU, D’Amico TA. Improving lung cancer outcomes by improving the quality of surgical care. Transl Lung Cancer Res 2015;4:424-31.
- Sroufe R, Kong FM. Triaging early stage lung cancer patients into non-surgical pathways: who, when, what? Transl Lung Cancer Res 2015;4:438-47.
- Osarogiagbon RU, Cappuzzo F, Ciuleanu T, et al. Erlotinib therapy after initial platinum doublet therapy in patients with EGFR wild type non-small cell lung cnacer: results of a combined patient-level analysis of the NCIC CTG BR.21 and SATURN trials. Transl Lung Cancer Res 2015;4:465-74.
- Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community healthcare setting. Transl Lung Cancer Res 2015;4:456-64.
- Ju F, Lee HK, Osarogiagbon RU, et al. Computer modeling of lung cancer diagnosis-to-treatment process. Transl Lung Cancer Res 2015;4:404-14.