A meta-analysis of safety and efficacy on first-line S-1 therapy in cancer patients
Introduction
S-1 is an oral fluoropyrimidine anticancer drug, combined with tegafur (prodrug of fluorouracil), gimeracil (dihydropyrimidine dehydrogenase inhibitor), and oteracil in a molar ratio of 1:0.4:1. It has been demonstrated to be beneficial in the treatment of many types of metastatic cancers, including advanced gastric cancer (AGC), pancreatic cancer, non-small cell lung cancer (NSCLC), colorectal cancer, biliary tract cancer, head and neck cancer and so on (1-5). Recent studies also showed that S-1 can reduce the gastrointestinal (GI) toxic effects of fluorouracil. Since the 1990s, S-1 has been used for the treatment of many types of cancers. The SAMIT, a phase 3 factorial randomized controlled trial (RCT), indicated that patients with T4a or T4b gastric cancer who were treated with S-1 therapy were superior to tegafur and uracil (UFT), therefore for locally AGC S-1 monotherapy should remain the standard treatment in Japan (6). When treated as a single agent, this drug, with high overall and relapse-free survival rates at 3 years and low incidence of adverse effects, was feasible for postoperative lung cancer patients (5).
Previous meta-analyses, which were conducted to investigate the prognostic significance of S-1-based therapy vs. S-1 monotherapy in patients with AGC, have showed that there were significantly longer median overall survival (OS) time and median progression-free survival (PFS) time in AGC patients receiving S-1-based therapy, on the other hand, the incidence of grade 3/4 neutropenia was higher in S-1-based therapy (7). However, in that meta-analysis, the sample size was relatively small and they only compared S-1-based therapy vs. S-1 monotherapy in AGC patients, and we believe that those findings should be confirmed with larger studies and other tumor types. To evaluate the incidence of high grade adverse effects and the efficacy of S-1-based therapy vs. S-1 monotherapy in cancer patients, we conducted an updated systematic review and meta-analysis with the aim of investigating whether S-1 monotherapy is low toxic and S-1-based therapy is more effective than S-1 monotherapy in cancer patients.
Material and methods
Search strategy
We searched the electronic databases, including PubMed, Embase, and Cochrane database. The upper date limit was March 2015, with no lower date limit. Searches include the terms: (“S-1”) and (“cancer”, OR “carcinoma”) and (“clinical trial”, OR “randomized controlled trial”). The reference lists of included studies were also searched.
Eligibility criteria
The eligibility criteria for this meta-analysis are: (I) prospective phase 2 and 3 clinical trials in cancer patients; (II) the language restricted to English; (III) presented the main adverse events data of S-1 therapy; (IV) participants assigned to first-line treatment with single agent S-1 at 80-120 mg/day twice daily (the daily dose was assigned according to body surface area as follows: <1.25 m2, 80 mg daily; ≥1.25-<1.5 m2, 100 mg daily; and ≥1.5 m2, 120 mg daily) on 4 weeks of a 6-week circle or 2 weeks of a 3-week circle; (V) if the same study was published in several publications, we only included the most recent, or complete. Phase I studies were excluded because of the different drug dosage and the relatively small number of patients on these trials. Two reviewers independently assess each study for inclusion.
Data extraction and quality assessment
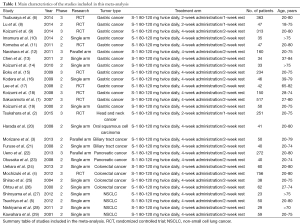
Two reviewers independently extracted information from included studies using a traditionalized format, and a third reviewer verified them. Information collected included: first author, publishing year, trial phase, research type, tumor type, treatment arm, sample size, dosage of S-1, and the number of adverse effects (Table 1). And for studies with S-1-based therapy, we also collected the median OS and PFS, and the hazard ratio (HR) of PFS or OS and its 95% confidence interval (CI).
Full table
Two independent researchers conducted quality assessment of included studies using the Newcastle-Ottawa Quality Assessment Scale for case control studies and for cohort studies (30). All of the studies included had a high quality with more than five stars each one.
Data analysis
For each study, we calculated the proportion and 95% CI of the majority grade 3/4 adverse effects in cancer patients treated with S-1 monotherapy or S-1-based therapy. For studies with S-1-based treatment in the same trial, we also calculated and compared the relative risk (RR) of grade 3/4 adverse effects and the HR and its 95% CI of median OS and PFS [two (12,22) of them was derived via the methods developed by Parmar et al. (31)]. Heterogeneity for studies was calculated using the χ2-based Q statistic. If P<0.05 or I2>50%, we could consider that there was statistically significant heterogeneity. Then data were analyzed using a random effects model. The publication bias was performed using the Begg’s and Egger’s tests (32,33). All of the data from included studies were pooled using Stata version 12.0.
Results
Study selection and characteristics
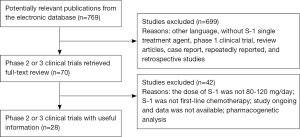
A total of 769 references were yielded from initial searches of the electronic database. Then 699 titles and abstracts were filtered out based on the inclusion criteria. Another 42 articles were excluded after a full-text review. Finally, we included 28 studies (19 phase 2 and 9 phase 3) comprising 2,359 participants. The flow chart of this meta-analysis is described in Figure 1.
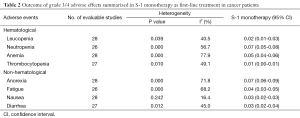
Incidence of high-grade adverse events
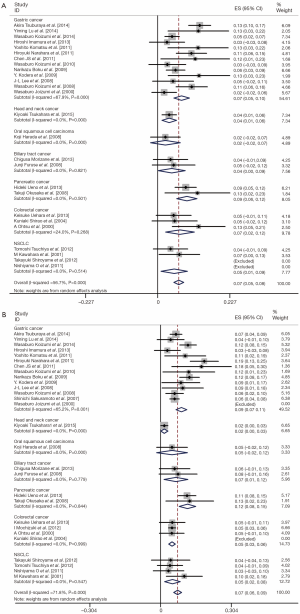
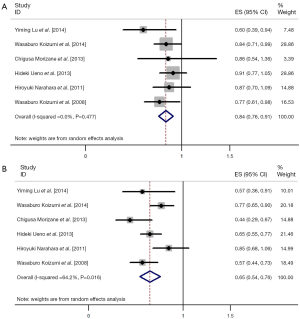
All of the 28 studies provided grade 3/4 adverse events (Table 2). The total number of patients included was 2,359. No data for neutropenia and fatigue were available in two studies [(1,4); (5,27) respectively]; no data for thrombocytopenia and diarrhea were available in one study [(15); (22) respectively]. Pooled data from these studies demonstrated a grade 3/4 adverse events rate of 32% for S-1 monotherapy. In addition, the grade 3/4 hematological event rate was 15% and the grade 3/4 non-hematological event rate was 17% for S-1 monotherapy as first-line treatment. The results of the meta-analysis for neutropenia and anorexia were shown in Figure 2. The incidence of grade 3/4 neutropenia ranged from 0 to 13%; the highest incidence was noted in a phase 3 RCT with gastric cancer (6), and the lowest incidence was observed in patients with NSCLC (27,28). However, the incidence of grade 3/4 anorexia ranged from 0 to 19%; the highest incidence was noted in a randomized phase 3 study with gastric cancer (12), and the lowest incidence was observed in patients with gastric cancer and colorectal cancer (19,25). This meta-analysis exhibited a significant heterogeneity among included studies (I2=56.7%, P=0.00 for neutropenia and I2=71.8%, P=0.00 for anorexia), and the calculated summary incidence of grade 3/4 neutropenia and anorexia among patients receiving S-1 was respectively 7% (95% CI: 5-8%) and 7% (95% CI: 6-9%) using a random effects model (Figure 2).
Full table
Subgroup analysis according to tumor type
To reduce the influence of significant heterogeneity, we carried out a subgroup analysis to confirm whether the tumor type had an influence on the incidence of high-grade adverse events with S-1 monotherapy. There was no significant difference of occurrence of grade 3/4 neutropenia and anorexia between gastric cancer, colorectal cancer and NSCLC; in pancreatic cancer, they were a little bit higher (Figure 2).
Difference between S-1-based therapy and S-1 monotherapy
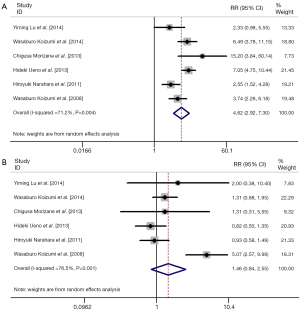
We also performed meta-analysis to derive a more accurate estimate of the prognostic value of S-1 based therapy vs. S-1 monotherapy in tumor patients (Figures 3,4). We found that the tumor patients who receiving S-1 based therapy had longer median OS time and median PFS time than those who receiving S-1 monotherapy (HR 0.836, 95% CI: 0.761-0.911, P=0.000, and HR 0.650, 95% CI: 0.540-0.759, P=0.000) (Figure 3). On the other hand, the RR of neutropenia and anorexia were respectively 4.62 (95% CI: 2.92-7.30) and 1.46 (95% CI: 0.84-2.55) (Figure 4). The incidence of grade 3/4 neutropenia was higher in patients with S-1-basedtherapy than those in S-1 monotherapy.
Publication bias
We performed Begg’s funnel plot and Egger’s test to evaluate the publication bias of the eligible studies. No publication bias was detected by either the funnel plot or Egger’s test of grade 3/4 proteinuria neutropenia (P=0.913 and P=0.418) and anorexia (P=0.300 and P=0.840) (Figure 5).

Discussion
Since the 1990s, S-1 has been used for the treatment of many types of cancers. Recently a meta-analysis compared S-1 with 5-Fu (34). 5-Fu has been a main anticancer agent for malignancies since it was introduced in 1957 (35). Their meta-analysis demonstrated that there were statistically significant improvements of PFS and ORR in the S-1-based chemotherapy in patients with AGC (P<0.001; P=0.005). S-1 has remarkable survival benefits, and S-1-based chemotherapy could replace 5-Fu-based therapy in advanced GI cancer in Asian patients (34).
The aim of this study is to evaluate the incidence of grade 3/4 adverse effects due to S-1 therapy and the efficacy of S-1-based therapy vs. S-1 monotherapy. We conducted this updated systematic review and meta-analysis to investigate whether S-1 monotherapy is low toxic and S-1-based therapy is more effective than S-1 monotherapy in cancer patients. The meta-analysis included 28 studies, including 19 phase 2 trials and 9 phase 3 trials. Our results showed that first-line S-1 monotherapy had low incidence of grade 3/4 adverse effects. The highest rate grade 3/4 hematological event was neutropenia (7%, 95% CI: 5-8%); the highest rate grade 3/4 non-hematological event was anorexia (7%, 95% CI: 6-9%). In addition, there was no significant difference of occurrence of grade 3/4 neutropenia and anorexia between gastric cancer, colorectal cancer and NSCLC; in pancreatic cancer, they were a bit higher.
We also investigated the efficacy of S-1-based therapy vs. S-1 monotherapy. The results of our meta-analysis showed that longer OS time and PFS time was exhibited in S-1-based therapy, compared with S-1 monotherapy (HR 0.836, 95% CI: 0.761-0.911, P=0.000, and HR 0.650, 95% CI: 0.540-0.759, P=0.000, respectively). However, the incidence of grade 3/4 adverse effects was also higher in S-1-based therapy than S-1 monotherapy in cancer patients, with RR of neutropenia and anorexia were respectively 4.62 (95% CI: 2.92-7.30) and 1.46 (95% CI: 0.84-2.55).
Our meta-analysis confirmed the previous analysis by Wu et al. (7), which also found significantly longer median OS time and median PFS time in AGC patients receiving S-1-based therapy compared with S-1 monotherapy (P=0.000 and P=0.015, respectively), with higher incidence of grade 3/4 neutropenia and anemia.
There are also several limitations in our meta-analysis. Firstly, the heterogeneity was statistically significant in the primary studies. The main reasons may be that definition of the type and grade for adverse events may be different by different investigators and the clinical trial design and modes of treatment may be different. Secondly, a majority of eligible studies were not RCT. The PFS and OS of S-1 monotherapy should be compared with control group with placebo in high-quality RCTs. Thirdly, the HR and CI of OS and PFS in two studies were derived from the methods developed by Parmar et al. (31). In some ways, this estimate method may influence the calculated HRs and their CIs. Finally, all of the studies included were in East Asia, including Japan, China, and Korea. The conclusion should be confirmed in Western studies.
In summary, our meta-analysis firstly estimated the high grade adverse effects of S-1 monotherapy in cancer patients, including gastric cancer, pancreatic cancer, NSCLC, colorectal cancer, biliary tract cancer, head and neck cancer and so on. S-1 monotherapy was demonstrated with low incidence of high grade adverse effects, therefore it is well tolerated for majority cancer patients. On the other hand, S-1-based therapy significantly improved OS and PFS compared with S-1 monotherapy, with an increased risk of high grade adverse effects. When the adverse effects can be tolerated, the treatment of S-1-based therapy is better than S-1 monotherapy. Our results should be confirmed with larger RCTs.
Acknowledgements
Funding: This study was supported by the Natural Science Foundation of Jiangsu Province (NO. BK2011658), Clinical Science and Technology Project of Jiangsu Province (NO. BL2013026) and The National Natural Science Foundation of China (NO. 81302032).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [PubMed]
- Tsukahara K, Kubota A, Hasegawa Y, et al. Randomized phase III trial of adjuvant chemotherapy with S-1 after curative treatment in patients with squamous-cell carcinoma of the head and neck (ACTS-HNC). PLoS One 2015;10:e0116965. [PubMed]
- Morizane C, Okusaka T, Mizusawa J, et al. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci 2013;104:1211-6. [PubMed]
- Mochizuki I, Takiuchi H, Ikejiri K, et al. Safety of UFT/LV and S-1 as adjuvant therapy for stage III colon cancer in phase III trial: ACTS-CC trial. Br J Cancer 2012;106:1268-73. [PubMed]
- Tsuchiya T, Nagayasu T, Yamasaki N, et al. A multicenter phase II study of adjuvant chemotherapy with oral fluoropyrimidine S-1 for non-small-cell lung cancer: high completion and survival rates. Clin Lung Cancer 2012;13:464-9. [PubMed]
- Tsuburaya A, Yoshida K, Kobayashi M, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol 2014;15:886-93. [PubMed]
- Wu JR, Tang WZ, Chen X, et al. S-1-based therapy versus S-1 monotherapy in advanced gastric cancer: a meta-analysis. Tumour Biol 2014;35:3283-93. [PubMed]
- Lu Y, Liu Z, Zhang J. S-1 plus oxaliplatin vs. S-1 as first-line treatment in patients with previously untreated advanced gastric cancer: a randomized phase II study. J Chemother 2014;26:159-64. [PubMed]
- Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 2014;140:319-28. [PubMed]
- Imamura H, Kishimoto T, Takiuchi H, et al. Phase II study of S-1 monotherapy in patients over 75 years of age with advanced gastric cancer (OGSG0404). J Chemother 2014;26:57-61. [PubMed]
- Komatsu Y, Takahashi Y, Kimura Y, et al. Randomized phase II trial of first-line treatment with tailored irinotecan and S-1 therapy versus S-1 monotherapy for advanced or recurrent gastric carcinoma (JFMC31-0301). Anticancer Drugs 2011;22:576-83. [PubMed]
- Narahara H, Iishi H, Imamura H, et al. Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer 2011;14:72-80. [PubMed]
- Chen JS, Chao Y, Hsieh RK, et al. A phase II and pharmacokinetic study of first line S-1 for advanced gastric cancer in Taiwan. Cancer Chemother Pharmacol 2011;67:1281-9. [PubMed]
- Koizumi W, Akiya T, Sato A, et al. Phase II study of S-1 as first-line treatment for elderly patients over 75 years of age with advanced gastric cancer: the Tokyo Cooperative Oncology Group study. Cancer Chemother Pharmacol 2010;65:1093-9. [PubMed]
- Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063-9. [PubMed]
- Kodera Y, Ito S, Mochizuki Y, et al. A phase II study of radical surgery followed by postoperative chemotherapy with S-1 for gastric carcinoma with free cancer cells in the peritoneal cavity (CCOG0301 study). Eur J Surg Oncol 2009;35:1158-63. [PubMed]
- Lee JL, Kang YK, Kang HJ, et al. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer 2008;99:584-90. [PubMed]
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. [PubMed]
- Koizumi W, Kurihara M, Nakano S, et al. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 2000;58:191-7. [PubMed]
- Harada K, Sato M, Ueyama Y, et al. Multi-institutional phase II trial of S-1 in patients with oral squamous cell carcinoma. Anticancer Drugs 2008;19:85-90. [PubMed]
- Furuse J, Okusaka T, Boku N, et al. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 2008;62:849-55. [PubMed]
- Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640-8. [PubMed]
- Okusaka T, Funakoshi A, Furuse J, et al. A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 2008;61:615-21. [PubMed]
- Uehara K, Maeda A, Sakamoto E, et al. Phase II trial of adjuvant chemotherapy with S-1 for colorectal liver metastasis. Ann Surg Oncol 2013;20:475-81. [PubMed]
- Shirao K, Ohtsu A, Takada H, et al. Phase II study of oral S-1 for treatment of metastatic colorectal carcinoma. Cancer 2004;100:2355-61. [PubMed]
- Ohtsu A, Baba H, Sakata Y, et al. Phase II study of S-1, a novel oral fluorophyrimidine derivative, in patients with metastatic colorectal carcinoma. S-1 Cooperative Colorectal Carcinoma Study Group. Br J Cancer 2000;83:141-5. [PubMed]
- Shiroyama T, Kijima T, Komuta K, et al. Phase II tailored S-1 regimen study of first-line chemotherapy in elderly patients with advanced and recurrent non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:783-9. [PubMed]
- Nishiyama O, Taniguchi H, Kondoh Y, et al. Phase II study of S-1 monotherapy as a first-line treatment for elderly patients with advanced nonsmall-cell lung cancer: the Central Japan Lung Study Group trial 0404. Anticancer Drugs 2011;22:811-6. [PubMed]
- Kawahara M, Furuse K, Segawa Y, et al. Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 2001;85:939-43. [PubMed]
- Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [PubMed]
- Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335-71. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Cao C, Zhang X, Kuang M, et al. Survival benefit from S-1 as compared to Fluorouracil in Asian patients with advanced gastrointestinal cancer: a meta-analysis. Cancer Sci 2014;105:1008-14. [PubMed]
- Heidelberger C, Chaudhuri NK, Danneberg P, et al. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957;179:663-6. [PubMed]