
Vimentin expression status is a potential biomarker for brain metastasis development in EGFR-mutant NSCLC patients
Introduction
Non-small cell lung cancer (NSCLC) poses a major health problem throughout the world and also in Thailand (1,2). Only 19% of all patients with lung cancers are alive 5 years or more after diagnosis (3). Despite newer targeted agents improving the systemic control of malignancy and hence survival, the incidence of brain metastasis (BM) has still increased. Approximately 10% of NSCLC patients develop BM at presentation, and approximately 40% of all patients develop BM subsequently (4). Incidence is higher in patients, especially whose cancers harbor epidermal growth factor receptor (EGFR) mutation, in whom up to 50–60% will develop BM over the course of their disease (5-8). The association between the EGFR mutation status and BM in patients with NSCLC has been reported (5,8). Patients with EGFR mutations were more likely to develop BM than those with EGFR wild type, especially during the course of the disease (9). It remains unclear whether this is because these patients have longer survival times and thus, more time to develop BM, whether there are selective pressure and poor central nervous system (CNS) penetration of systemic therapies, or whether these mutation-driven cancers have biologic features that predispose towards progression and growth within the CNS. Previous studies on risk factors for the development of BM in NSCLC including younger age (8,10-13), female gender (12), non-squamous cell carcinoma (11-14), and more advanced in tumor and nodal stage (8,10,11,14) have been reported, however, most of these studies included unselected patients with NSCLC. Biomarker prediction for BM in these populations is not well studied.
Several studies suggested that molecular factors play an important role in contributing to BM, such as genes involved in cell adhesion, extravasation, metabolism, and cellular signaling (15). Epithelial mesenchymal transition (EMT), a process by which epithelial cells lose their cell polarity and cell-cell adhesion and gain migratory and invasive properties to become mesenchymal stem cells, play a role in the initiation of metastasis. Accumulating evidence has indicated that vimentin is critical for the progression and prognosis of lung cancer (16). Furthermore, activation of EGFR expression promoted EMT phenotype in various cancer cell lines, including lung cancer (17,18). Although the correlation between EGFR mutation and BM has been widely studied (9,13,19,20), data concerning the association of EMT status and BM development are scare and underlying mechanisms of BM progression in these patients remain poorly understood.
Therefore, we aimed to identify the factors associated with BM in EGFR-mutant NSCLC and identify the patients at higher risk for BM development for earlier detection and treatment as well as characterizing the role of EMT marker as a biomarker that can predict the occurrence of BM in EGFR-mutant NSCLC. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-1020).
Methods
Study population
This retrospective study enrolled patients who were diagnosed with recurrent/metastatic NSCLC at King Chulalongkorn Memorial Hospital (KCMH) over a period of 5 years (January 1, 2013, to December 31, 2017) and had complete patient medical records on key exposure and outcome variables. The main inclusion criteria were adults aged 18 or older with cytology or histologically confirmed NSCLC who had EGFR testing results. The patients were excluded if they had more than one primary cancer, unknown EGFR mutation status, had anaplastic lymphoma kinase (ALK) rearrangement or other mutations, and incomplete follow up data. The presence of BM was confirmed by brain radiography, either by computed tomography (CT) or magnetic resonance imaging (MRI). Patients were categorized into initial BM (inBM) if BM was identified at presentation and subsequent BM (subBM) if BM was identified in patients who had negative brain imaging at baseline and were imaged to identify BM when BM associated neurologic symptoms/signs occurred or BM found during or after treatment. The variables include age, gender, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking status, histology, initial stage at diagnosis, number of metastatic sites, EGFR mutation subtypes, and treatment history were collected. EGFR mutations (including G719X in exon 18, exon 19 deletion, T790M in exon 20, and L858R and L861Q in exon 21) were performed by cobas®EGFR Mutation Test v2 kit according to the manufacture’s protocol. This study was approved by the Institutional Review Board of the Faculty of Medicine at Chulalongkorn University. (No. 267/62). For this retrospective study, the written informed consent from patients was waived per the IRB, and the study was performed following the Health Insurance Portability and Accountability Act and the Declaration of Helsinki (as revised in 2013).
Immunohistochemistry
Formalin-fixed and paraffin-embedded (FFPE) tumor samples from the histopathological files of the Department of Pathology, KCMH were retrospectively analyzed. Two-micron thick FFPE tissue sections on charged glass slides were prepared per standard protocol for IHC. Epitope retrieval was performed on the Dako PT link (Dako, Denmark) and immunostaining was performed using automated staining systems, DakoAutostainer Link48 (Dako, Denmark) with antibodies against vimentin antibody (Monoclonal Mouse anti-Human Vimentin, clone V9, RTU, Cat no; IR630, Dako Denmark). In accordance with similar thresholds used in previous studies (21), a value of ≥10% positive tumor cells independent of intensity was chosen to define positive expression of vimentin . Briefly, the staining intensity was determined by cytosolic staining for vimentin. Vimentin expression that was equal to or more than 10% of tumor cells with cytoplasmic staining intensity of +1, +2 or +3 was categorized as positive. All slides were evaluated by a pathologist (K.R) who was blinded from patient outcomes.
Statistical analysis
Categorical variables were summarized by frequencies and percentages while continuous variables were reported by the median and interquartile range (IQR). Clinicopathologic factors and treatment outcomes were analyzed in correlation with BM status using Chi-square or Fisher exact test as appropriate. The univariate and multivariate analysis assessed factors associated with the development of BM and analyzed by odds ratio (OR). Overall survival (OS) was defined from the date of recurrent or metastatic NSCLC diagnosis to the date of death or the last contact. Patients who were not deceased were censored on December 31, 2019. Time-to-event was analyzed using the Kaplan-Meier method and was compared between groups by the log-rank test. Hazard ratios (HR) and corresponding 95% confidence intervals (95% CI) were calculated. The P value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using GraphPad Prism version 8.00 for Windows (GraphPad Software, La Jolla, California, USA) and SPSS 23.0 (SPSS Inc, Chicago, Illinois, USA).
Results
Patient characteristics of the study population
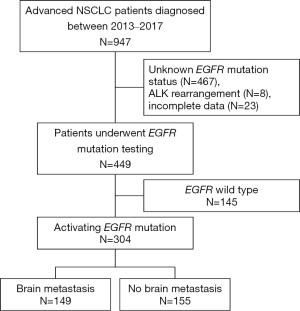
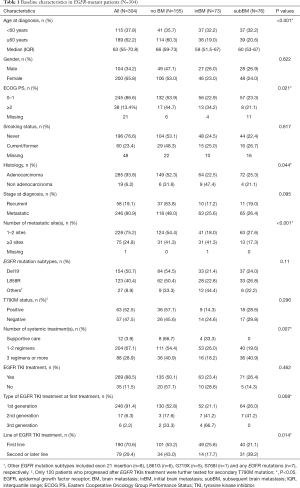
449 patients were identified. Of these, 304 patients (67.7%) who had EGFR mutations were analyzed (Figure 1). Baseline characteristics are summarized in Table 1. The median age of the patients was 63 years (IQR 55–70.8), mostly female (65.8%), good ECOG PS 0 to 1 (86.8%) and never smokers (76.6%). The majority of the patients were diagnosed as adenocarcinoma (93.8%), metastatic disease at diagnosis (80.9%), and 1 or 2 metastatic sites (75.2%). Exon 19 deletion (n=154) and L858R mutation (n=123) were the most common EGFR mutation subtypes, accounting for 91.1% of the patients. Other EGFR mutation subtypes included exon 21 insertion (n=8), L861G (n=6), G719X (n=5), S768I (n=1) and any EGFR mutations (n=7), respectively. Sixty-seven percent of patients received 1 or 2 lines of systemic treatment for the advanced stage of the disease. Of these, 269 patients (88.5%) received EGFR-TKIs during the course of the disease and 190 patients (70.6%) were treated with EGFR-TKIs as first-line treatment. 91.4% of patients received 1st generation EGFR TKI as the first EGFR TKI treatment. A total of 120 patients (45.6%) who progressed after EGFR-TKIs treatment were further tested for secondary T790M mutation and 63 patients (52.5%) were found to have the T790M mutation. Of these, 58 patients received osimertinib, 3rd generation of EGFR TKIs, as subsequent treatment.
Full table
Factors associated with the development of BM in patients with EGFR-mutant NSCLC
The median follow-up was 46.42 months (95% CI, 41.34–51.51), 73 patients (24%) experienced BM at diagnosis and 76 patients (25%) developed subBM. Baseline characteristics are reported in Table 1.
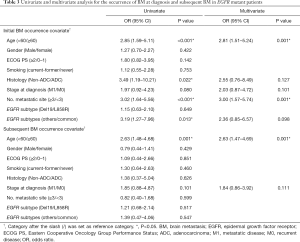
The clinicopathological factors that were significantly associated with the overall occurrence of BM included age <60 years (OR 2.74, 95% CI, 1.69–4.43, P<0.001), metastatic disease at diagnosis (OR 1.91, 95% CI, 1.05–3.45, P=0.032), and 3 or more metastatic sites (OR 1.69, 95% CI, 0.99–2.87, P=0.05). Multivariate analyses showed that only age <60 years was statistically significantly associated with BM occurrence. However, there was no difference in BM occurrence between the exon 19 deletion and L858R mutation (Table 2).

Full table
Interestingly, factors associated with the development of BM in patients with EGFR-mutant NSCLC differed between those who experienced BM at diagnosis and those who developed BM subsequently. EGFR-mutant patients who had inBM were more likely to be younger (<60 years) (OR 2.85, 95% CI, 1.59–5.11, P<0.001), with non-adenocarcinoma histology (OR 3.49, 95% CI, 1.19–10.21, P=0.022), 3 or more metastatic sites (OR 3.02, 95% CI, 1.64–5.56, P<0.001) and had uncommon EGFR mutation subtype (OR 3.19, 95% CI, 1.27–7.96, P=0.013) compared to patients without BM. Multivariate analyses revealed that younger patients (<60 years) (OR 2.81, 95% CI, 1.51–5.24, P=0.001) and higher disease burden (≥3 metastatic sites) (OR 3.00, 95% CI, 1.57–5.74, P=0.001) were statistically significantly associated with inBM development. While only age <60 years (OR 2.63, 95% CI, 1.47–4.69, P=0.001) was associated with subBM compared to those without BM (Table 3).
Full table
Systemic treatment may contribute to the occurrence of subBM in patients with EGFR-mutant NSCLC
We analyzed whether systemic treatment is associated with subBM occurrence. Among 231 patients with EGFR-mutated NSCLC without BM at diagnosis, 223 patients (96.5%) received systemic treatment for the advanced stage of the disease. Of these, 206 patients (89.2%) received EGFR-TKIs during the course of the disease and 141 patients (68.4%) were treated with EGFR-TKIs as first-line treatment. 54 of 101 patients who progressed after EGFR-TKIs treatment were found to have secondary T790M mutation and 57 patients received 3rd generation of EGFR TKIs as subsequent treatment.
Patients who received 3 lines or more of systemic treatment (OR 2.84, 95% CI, 1.58–5.12, P<0.001) and did not receive EGFR-TKI as first-line treatment (OR 2.30, 95% CI, 1.25–4.23, P=0.007) were associated with subBM. However, these treatment factors were not statistically significantly associated with subBM occurrence after adjusting for other clinicopathological factors (Table S1). Outcomes of EGFR-TKI were also analyzed by the time to subBM (TTSBM). Treatment of EGFR-TKIs had longer time to subBM than those patients who did not receive EGFR-TKIs (median TTSBM was 51.78 months vs. 26.61 months, P=0.002; Figure S1). Cox regression analysis was performed on the factors that would correlate with TTSBM. Multivariate analyses revealed that the treatment of EGFR TKIs could delay the occurrence of subBM more than those who did not receive EGFR-TKIs (HR 2.18, 95% CI, 1.18–4.02, P=0.013; Table S2).
Outcome of BM in patients with EGFR-mutant NSCLC
At data cut-off on December 31, 2019, the median follow-up time was 46.42 months (95% CI, 41.34–51.51) and 80 patients (26.3%) survived to the last contact. The median OS of the overall study cohort was 22.97 months (95% CI, 20.98–24.95). Patients with BM had a significantly shorter OS than patients without BM (median OS was 22.44 months (95% CI, 19.76–25.12) vs. 24.18 months (95% CI, 20.41–27.95), respectively; HR 1.48; 95% CI, 1.14–1.93, P=0.004; Figure 2A).
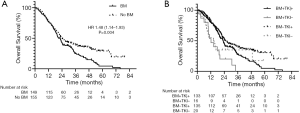
Of the 304 patients with EGFR-mutant NSCLC, 268 patients (88.2%) received EGFR TKIs whereas 36 patients (11.8%) did not. Patients without BM who received EGFR TKIs had longer survival than those with BM who received EGFR TKIs, and those with/without BM but not received EGFR TKIs (median OS were 25.13, 22.96, 12.22 and 14.39 months, respectively; P<0.001; Figure 2B).
Vimentin expression status as one of EMT marker predicts the development of BM in patients with EGFR-mutant NSCLC
To validate whether vimentin expression status as a predictive marker for BM occurrence, we next analyzed vimentin expression by IHC on 190 available tumor specimens according to EGFR mutation status. Baseline characteristics of these 190 patients are listed in Table S3.
Overall, the mean of vimentin expression in our study was 28.5% and the distribution of vimentin expression according to BM status and EGFR mutation status was shown in Figure S2. Using a value of ≥10% positive tumor cells, vimentin expression was detected in 83 patients (43.7%) and was found more common in patients with BM than those without BM (53.6% vs. 33.3%, respectively; P=0.005). Although vimentin expression was similar between mutated-EGFR (40.5%) and those with wild-type EGFR (49.3%), respectively, we found a significant association between vimentin expression and the occurrence of BM in patients with EGFR-mutant NSCLC (52.4% vs. 27.6%, respectively; P=0.006), but not in those with wild type EGFR (55.9% vs. 42.9%, respectively; P=0.28; Figure S3).
Using multivariate analysis in patients with EGFR-mutant NSCLC, the occurrence of BM was significantly associated with the expression of vimentin (OR 2.53, 95% CI, 1.11–5.77; P=0.027; Table 4). Moreover, vimentin expression also was statistically significantly associated with subBM occurrence (OR 3.06, 95% CI, 1.15–8.11, P=0.025) and there was a trend of association with inBM occurrence (OR 2.69, 95% CI, 0.88–8.17, P=0.08; Table 4). Conversely, there was no association were identified between the vimentin expression and the BM occurrence in those with wild-type EGFR (Table S4).
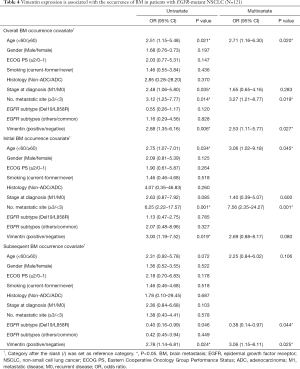
Full table
Furthermore, additional analysis using other methods for cut-off point of vimentin expression by receiver operating characteristic (ROC) analysis was also done. The area under the curve (AUC) was 0.604 (95% CI, 0.523–0.685) and the cut-off values were 22.5%. Consistent with our results that using cut-off point at 10% of positive cells, vimentin expression in EGFR-mutant but not WT significantly correlated with occurrence of BM (Figure S4, Table S5).
Taken together, these findings indicate that vimentin expression plays a role in the initiation of metastasis and promotes BM occurrence especially in patients with EGFR-mutant NSCLC, and maybe serves as a potential biomarker for predicting BM occurrence in these patients.
Prognostic role of vimentin expression in patients with NSCLC
To determine the prognostic role of vimentin expression, we next analyzed the correlation of vimentin expression and OS according to BM and EGFR mutation status.
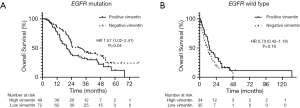
In the overall population regardless of BM status, tumors with positive vimentin expression tended to have shorter OS compared to those with negative expression. Median OS was 19.7 months (95% CI, 14.23–25.19) in vimentinpositive and 22.7 months (95% CI, 20.43–24.98) in vimentinnegative, respectively (P=0.193). However, tumors with vimentinpositive correlated with survival according to EGFR mutation status. In the EGFR-mutant group, tumors with vimentinpositive had a significantly shorter OS than those with negative expression (median OS was 20.0 months (95% CI, 14.51–25.51) vs. 30.9 months (95% CI, 20.99–40.84), respectively; HR 1.57; P=0.04). (Figure 3A) While there was a similar OS between positive- and negative-vimentin expression in the wild type-EGFR group. [median OS was 14.29 months (95% CI, 10.16–18.43 months) vs. 11.86 months (95% CI, 7.17–16.55 months), respectively; HR 0.70; P=0.18; Figure 3B]. Moreover, in EGFR-mutant group, patients with BM and vimentinpositive was the worse OS compared to patients with BM and vimentinnegative, and patients without BM and positive/negative vimentin expression (median OS were 16.27, 23.76, 50.40 and 38.05 months, respectively; P<0.001).
Discussion
Our study found a high incidence of BM in patients with EGFR-mutant NSCLC (49%). Similar results were reported by previous studies (40–64%) (8,18,22-24). Furthermore, EGFR mutation not only was associated with overall BM but also predicted subBM (9,18,19,22). As previously mentioned, studies on risk factors for the development of BM in NSCLC have been reported (5,6-8,10-14,25-27), however, most of these studies included unselected patients with NSCLC and studies in EGFR-mutant patients were not well evaluated. Our study focused on the factors for the BM occurrence in patients with EGFR-mutant NSCLC and found the difference of risk factors between patients who experienced BM at diagnosis and patients who developed BM subsequently. In EGFR-mutant patients who had inBM were more likely to be younger (<60 years), had non-adenocarcinoma histology, high disease burden (≥3 metastatic sites), and uncommon EGFR mutation subtype whereas only age <60 years was associated with subBM compared to patients without BM. Moreover, systemic treatment may contribute to subBM in these patients. The high frequency of subBM in EGFR-mutated NSCLC can be mainly attributed to better response to systemic treatment which leads to longer survival and thus, probably increases the risk of subBM development. Our study also found an association between subBM occurrence and patients who received multiple lines of systemic treatment. We found that EGFR-TKIs could delay the occurrence of subBM more than patients who did not receive EGFR-TKIs.
It remains unclear whether the EGFR-mutation-driven cancers have biologic features that predispose towards progression and growth within the CNS. Accumulating evidence has indicated that vimentin is critical for the progression and prognosis of lung cancer (16) and preclinical studies suggested that the activation of EGFR expression promoted EMT phenotype in various cancer cell lines, including lung cancer (17,18). Activating EGFR mutation enhances cell mobility and promotes vimentin expression, a hallmark of mesenchymal cells. The analyses of tumor samples revealed the association between EGFR mutation status and vimentin expression (18). Recently, AXL, a receptor tyrosine kinase belonging to the TAM (TYRO3/AXL/MER) family, and its ligand GAS6, growth arrest-specific gene 6, has been reported to have a potential key role in various processes, including epithelial to mesenchymal transition (28,29). High expression of AXL/GAS6 has been found to be poor prognostic biomarker for NSCLC patients with BM. However, the role of EMT marker in BM from patients with EGFR-mutant NSCLC and its potential prognostic importance have not been well identified. Herein, the analyses of tumor samples from our cohort supported the correlation of vimentin expression and BM occurrence in patients with EGFR-mutated NSCLC. Overexpression of vimentin has been observed in nearly 45% of patients in this study and found more common in patients with BM than those without BM. We also found the association between vimentin expression and the occurrence of BM especially in patients with EGFR-mutant NSCLC and conferred worse survival outcome in these patients. To best of our knowledge, this is the first study to identify the vimentin expression as one potential biomarker for poor outcome of BM among patients with EGFR-mutant NSCLC. Because this biomarker can be readily established in clinical practice using widely available IHC methods with reasonable cost, our findings may have clinical implications as this potential biomarker can predict BM occurrence and serve as a therapeutic target in these patients.
There are several limitations to our study. First, patient selection and information bias may have occurred due to the retrospective nature and single center setting of the study. Second, there was a different strategy to define patients with BM due to a lack of routine brain imaging in asymptomatic patients. Third, the possibility of vimentin expression discordance between the primary and metastatic sites may influence our results. There was a various cut-off point of IHC interpretation for vimentin expression when comparing our result to other studies. Therefore, interpretation should be done more carefully for future study. Finally, there are several markers related to EMT process, including epithelial markers (E-cadherin, N-cadherin), transcription factors that repress E-cadherin expression (Snail, Twist) and mesenchymal markers (vimentin) (30). Combination assessment of these EMT-related biomarkers to explore the clinical significance of distinct EMT phenotype in these patients would add additional information.
In conclusion, younger patients with EGFR-mutant NSCLC who had high disease burden were more likely to development of BM. Vimentin serves as a biomarker predicting BM and poor prognostic factor in EGFR-mutant patients. Our findings may have important implications for treatment and follow-up strategies in these high-risk patients. Vimentin may be a prognostic factor and therapeutic target for BM in patients with EGFR mutant NSCLC.
Acknowledgments
Funding: This work was supported by The Ratchadapiseksompotch Endowment Fund [RA62/101], Faculty of Medicine, Chulalongkorn University to PS; and Chulalongkorn Academic Advancement into Its 2nd Century (CUAASC) Project to VS and CV.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-1020
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-1020
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-1020). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the Faculty of Medicine at Chulalongkorn University. (No. 267/62). For this retrospective study, the written informed consent from patients was waived per the IRB, and the study was performed following the Health Insurance Portability and Accountability Act and the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 2004;45 Suppl 2:S253-7. [Crossref] [PubMed]
- Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195-9. [Crossref] [PubMed]
- Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012;118:4502-11. [Crossref] [PubMed]
- Li B, Sun SZ, Yang M, et al. The correlation between EGFR mutation status and the risk of brain metastasis in patients with lung adenocarcinoma. J Neurooncol 2015;124:79-85. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015;20:674-9. [Crossref] [PubMed]
- Li L, Luo S, Lin H, et al. Correlation between EGFR mutation status and the incidence of brain metastases in patients with non-small cell lung cancer. J Thorac Dis 2017;9:2510-20. [Crossref] [PubMed]
- Ceresoli GL, Reni M, Chiesa G, et al. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: risk factors analysis. Cancer 2002;95:605-12. [Crossref] [PubMed]
- Bajard A, Westeel V, Dubiez A, et al. Multivariate analysis of factors predictive of brain metastases in localised non-small cell lung carcinoma. Lung Cancer 2004;45:317-23. [Crossref] [PubMed]
- Hsiao SH, Chung CL, Chou YT, et al. Identification of subgroup patients with stage IIIB/IV non-small cell lung cancer at higher risk for brain metastases. Lung Cancer 2013;82:319-23. [Crossref] [PubMed]
- Ji Z, Bi N, Wang J, et al. Risk factors for brain metastases in locally advanced non-small cell lung cancer with definitive chest radiation. Int J Radiat Oncol Biol Phys 2014;89:330-7. [Crossref] [PubMed]
- Mujoomdar A, Austin JH, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 2007;242:882-8. [Crossref] [PubMed]
- Eichler AF, Chung E, Kodack DP, et al. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol 2011;8:344-56. [Crossref] [PubMed]
- Ye Z, Zhang X, Luo Y, et al. Prognostic Values of Vimentin Expression and Its Clinicopathological Significance in Non-Small Cell Lung Cancer: A Meta-Analysis of Observational Studies with 4118 Cases. PLoS One 2016;11:e0163162. [Crossref] [PubMed]
- Lo HW, Hsu SC, Xia W, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res 2007;67:9066-76. [Crossref] [PubMed]
- Hsiao SH, Chou YT, Lin SE, et al. Brain metastases in patients with non-small cell lung cancer: the role of mutated-EGFRs with an exon 19 deletion or L858R point mutation in cancer cell dissemination. Oncotarget 2017;8:53405-18. [Crossref] [PubMed]
- Ma X, Zhu H, Guo H, et al. Risk factors of brain metastasis during the course of EGFR-TKIs therapy for patients with EGFR-mutated advanced lung adenocarcinoma. Oncotarget 2016;7:81906-17. [Crossref] [PubMed]
- Hubbs JL, Boyd JA, Hollis D, et al. Factors associated with the development of brain metastases: analysis of 975 patients with early stage nonsmall cell lung cancer. Cancer 2010;116:5038-46. [Crossref] [PubMed]
- Richardson F, Young GD, Sennello R, et al. The evaluation of E-Cadherin and vimentin as biomarkers of clinical outcomes among patients with non-small cell lung cancer treated with erlotinib as second- or third-line therapy. Anticancer Res 2012;32:537-52. [PubMed]
- Han G, Bi J, Tan W, et al. A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget 2016;7:56998-7010. [Crossref] [PubMed]
- Matsumoto S, Takahashi K, Iwakawa R, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer 2006;119:1491-4. [Crossref] [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [Crossref] [PubMed]
- Hendriks LE, Smit EF, Vosse BA, Mellema WW, Heideman DA, Bootsma GP, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer 2014;84:86-91. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Baek MY, Ahn HK, Park KR, et al. Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med 2018;33:168-75. [Crossref] [PubMed]
- Linger RM, Keating AK, Earp HS, et al. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 2008;100:35-83. [Crossref] [PubMed]
- Shieh YS, Lai CY, Kao YR, et al. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia 2005;7:1058-64. [Crossref] [PubMed]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009;119:1429-37. [Crossref] [PubMed]


