
Complete disease remission in a TP53 and KRAS co-mutated brain oligometastatic lung cancer patient after immuno-chemotherapy and surgical resection: a case report
Introduction
Advanced non-small cell lung carcinoma (NSCLC) has a poor prognosis, with a 5-year overall survival (OS) rate of approximately 5%. Targeted therapy could contribute to improved survival for patients with actionable driver gene mutations but has no impact in driver gene-negative patients. With the application of immune checkpoint inhibitors (ICIs), the 5-year OS rate of advanced EGFR and ALK wild-type NSCLC has considerably improved. Results of a randomized phase I trial (KEYNOTE-001) suggest that pembrolizumab monotherapy provided durable antitumor activity, with a 23.2% and 15.5% increase in 5-year OS rates for treatment-naïve and previously treated advanced NSCLC patients, respectively. Furthermore, a randomized phase III trial (KEYNOTE-024) also demonstrated that the 5-year OS rate increased to 31.9% with pembrolizumab monotherapy in patients with advanced NSCLC with PD-L1 expression greater than 50%.
Oligometastasis disease, which is defined as the intermediate stage between locally advanced and widely disseminated disease, was proposed by Hellman and Weichselbaum in 1995 (1). The concept of oligometastatic lung cancer has been controversial; however, in 2019 a consensus statement was released with reference to the possibility of offering radical-intent treatment strategies. This consensus definition for oligometastatic lung cancer allowed a maximum of five metastases in up to three organs, without the presence of diffuse serosal metastases or bone marrow involvement (2). Furthermore, the incidence rate was about 4.0% for brain oligometastasis in NSCLC patients. Multimodal treatment is essential for oligometastatic lung cancer patients. A multicenter, phase II randomized study demonstrated that local consolidative therapy, in addition to systemic therapy, could significantly prolong the progression-free survival (PFS) and OS compared to systemic therapy alone (3). As a radical treatment, surgical resection is the most established local consolidative therapy, with previous studies showing that surgical therapy in oligometastatic lung cancer contributed to significantly better 5-year OS in various multimodal approaches (4). Combinations of ICI and chemotherapy may markedly increase response rate and survival which might allow oligometastatic lung cancer patients to become eligible for localized treatment modalities. Mutations of TP53 and KRAS are common in NSCLC patients, and previous study conducted the presence of TP53 and KRAS co-mutation has also been associated with response to checkpoint inhibitors. This report describes a brain oligometastatic lung cancer patient with TP53 and KRAS co-mutation who achieved a complete response after systemic immuno-chemotherapy and local surgical resection. As we known, this is the first reported case in which a brain oligometastatic NSCLC patient has achieved a complete response after immuno-chemotherapy plus local surgical resection. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-21-380).
Case presentation
A 62-year-old male with a 40-year smoking history was hospitalized following a one-month history of bloody sputum. Enhanced computed tomography (CT) indicated a mass with pleural invasion in the right upper lobe 3.5 cm × 3.3 cm in size (Figure 1A). Enhanced magnetic resonance imaging (MRI) showed five metastases (6.2 mm in right temporal lobe, 6.4 mm in right frontal lobe, 5.7 mm in left hippocampus, 9.5 mm in left parietal lobe and 17.9 mm in left temporal lobe) in the brain without edema as shown in Figure 1B. He has no any neurological symptoms. A CT-guided percutaneous biopsy for the lung mass was performed. Pathological examination showed that the right lung lesion was an invasive lung adenocarcinoma. The patient was diagnosed with oligometastatic Stage IVB lung adenocarcinoma (T2aN0M1c, IVB, AJCC 8th). We further performed next-generation sequencing with 68 cancer-related genes (Burning Rock Biotech, Guangzhou, China) where KRAS (p.Gly13Asp, 8.12%) and TP53 (p.Thr125Pro, 7.35%) mutations were detected. Immunohistochemistry with PD-L1 IHC 22C3 pharmDx assay (Dako) for PD-L1 expression was negative.
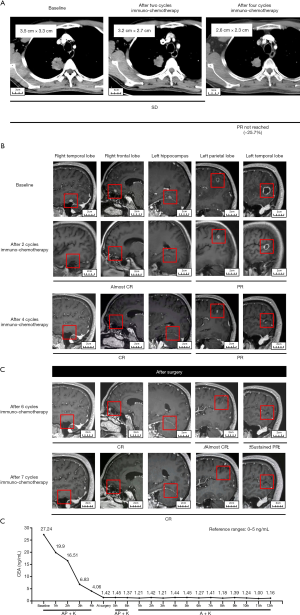
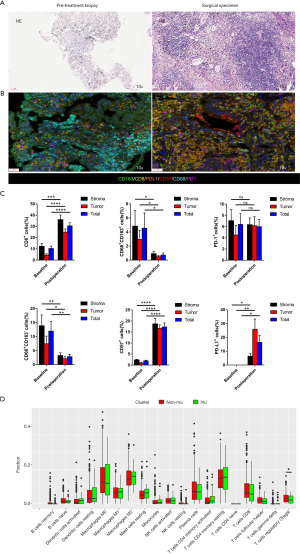
After the patient’s consent, the patient received four cycles of pemetrexed (500 mg/m2, d1), nedaplatin (75 mg/m2, d1) and pembrolizumab (100 mg, d1) every 21 days. After four cycles of systemic immuno-chemotherapy, a brain MRI indicated that three metastatic tumors had completely disappeared and the remaining two metastases had a marked partial response (PR) (Figure 1B). After four cycles of systemic immuno-chemotherapy, brain MRI indicated that three metastatic tumors had completely disappeared and the remaining two metastases had a marked PR status (Figure 1B). Chest CT showed that the right upper lobe lesion had approximately shrunk down by 25.7% in size but had not yet reached PR by Response Evaluation Criteria in Solid Tumors (RECIST) (Figure 1A). The only side effects during treatment were fatigue and gastrointestinal discomfort. The serum carcinoembryonic antigen (CEA) levels had significantly decreased to the normal range (Figure 1C). After careful multidisciplinary discussion and obtaining patient consent, we performed video-assisted right upper lung lobectomy plus regional lymphadenectomy. Pathologic results revealed that the pathological remission rate was 60% and all lymph node stations were negative. As shown in Figure 2A, widespread fibrosis and lymphocyte infiltration could be seen in the surgical specimen after immuno-chemotherapy. The patient received another two cycles of platinum-based immuno-chemotherapy after surgery, followed by maintenance therapy with pemetrexed and pembrolizumab. After seven cycles of maintenance therapy, MRI showed that all metastases in the brain could not be detected (Figure 1B); thus, the patient achieved a complete disease remission after systemic immuno-chemotherapy plus local surgical resection, with no sign of recurrence after 22 months. This study was approved by Ethics Committee of Tianjin Medical University General Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for specimen collection, genetic testing, use of this information for research purposes, and publication of the case report.
Discussion
As for advanced NSCLC patients, specifically for oligometastatic NSCLC patients, systematic therapy followed with local consolidative therapy could lead to a better PFS and OS according to the previous studies. To our knowledge, this is the first case in which a brain oligometastatic NSCLC patient has achieved a complete response after immuno-chemotherapy and complete surgical resection of the lung primary. Although long-term follow-up is necessary to evaluate the therapeutic effect of this treatment strategy, the PFS has been approximately two years. Since the best response for immunotherapy is complete response, and treatment was well-tolerated, previous evidence would suggest this patient may have good long-term survival (5). We would like to highlight possible reasons in this patient for the excellent and durable response despite baseline PD-L1 expression of 0%.
Previous data has indicated that ICI activity in cancer patients is linked to the magnitude of T-cell response within the tumor microenvironment (6). Schaer et al. have also demonstrated that pemetrexed chemotherapy could promote T-cell trafficking and infiltration of the tumor, with consequent enhanced effects of cancer immunotherapy (7). However, there are few studies concerning alteration of the immune microenvironment after systemic immuno-chemotherapy. As illustrated in Figure 2B,C, PD-L1 expression was dramatically increased, along with macrophage regression in the tumor after immuno-chemotherapy. It was also found that infiltration of CD8+ and NK cells was upregulated after immuno-chemotherapy. These results suggest that the underlying mechanism for disease remission in this patient was through immune activation enhanced by pemetrexed based chemotherapy. The dynamic nature of the PD-L1 upregulation with chemotherapy shown in this patient also illustrates the poor utility of static PD-L1 expression at baseline as a sole biomarker for use of ICI, and argues for better biomarkers for selection for treatment.
The presence of TP53 and KRAS co-mutation has also been associated with response to checkpoint inhibitors. Fang et al. reported that co-mutations of TP53 and KRAS served as potential biomarkers for response to PD-1 blockade immunotherapy in squamous-cell NSCLC (8). Dong et al. observed that TP53 or KRAS mutation in lung adenocarcinoma, especially those with co-occurring TP53/KRAS mutations, showed increased expression of PD-L1 and CD8+ T cells, along with marked clinical sensitivity to PD-1 inhibitors (9). In this study, we also investigated immune cell infiltration in TP53 and KRAS co-mutation in NSCLC using TCGA database and Cell type Identification By Estimating Relative Subsets Of RNA Transcripts (CIBERSORT). As shown in Figure 2D, TP53 and KRAS co-mutation was negatively correlated with infiltration of T regulatory cells (Tregs), which have been established as playing a role in the suppression of antitumor immunity (10). These findings indicate that TP53 and KRAS co-mutation constitute a “hot” immune microenvironment favoring anti-PD-1/PD-L1 immunotherapy.
In conclusion, we present a brain oligometastatic NSCLC patient with complete response after systemic immuno-chemotherapy and local surgical resection. This is a new mode of treatment for certain NSCLC patients with oligometastatic disease, which may achieve a better curative effect and survival. However, due to the complexity of the clinical diagnosis and individual difference, it is still uncertain whether this multimodality treatment regimen is suitable for all the oligometastatic NSCLC patients. Therefore, further investigation is worthwhile in this population, especially for patients with certain genetic mutations.
Acknowledgments
The authors appreciate the academic support from AME Lung Cancer Collaborative Group.
Funding: The present study was funded by the National Natural Science Foundation of China (No. 81772464).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-21-380
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-21-380). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by Ethics Committee of Tianjin Medical University General Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for specimen collection, genetic testing, use of this information for research purposes, and publication of the case report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Dingemans AC, Hendriks LEL, Berghmans T, et al. Definition of Synchronous Oligometastatic Non-Small Cell Lung Cancer-A Consensus Report. J Thorac Oncol 2019;14:2109-19. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- David EA, Clark JM, Cooke DT, et al. The Role of Thoracic Surgery in the Therapeutic Management of Metastatic Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1636-45. [Crossref] [PubMed]
- Gauci ML, Lanoy E, Champiat S, et al. Long-Term Survival in Patients Responding to Anti-PD-1/PD-L1 Therapy and Disease Outcome upon Treatment Discontinuation. Clin Cancer Res 2019;25:946-56. [Crossref] [PubMed]
- Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol 2021;18:842-59. [Crossref] [PubMed]
- Schaer DA, Geeganage S, Amaladas N, et al. The Folate Pathway Inhibitor Pemetrexed Pleiotropically Enhances Effects of Cancer Immunotherapy. Clin Cancer Res 2019;25:7175-88. [Crossref] [PubMed]
- Fang C, Zhang C, Zhao WQ, et al. Co-mutations of TP53 and KRAS serve as potential biomarkers for immune checkpoint blockade in squamous-cell non-small cell lung cancer: a case report. BMC Med Genomics 2019;12:136. [Crossref] [PubMed]
- Dong ZY, Zhong WZ, Zhang XC, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res 2017;23:3012-24. [Crossref] [PubMed]
- Persa E, Balogh A, Sáfrány G, et al. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Lett 2015;368:252-61. [Crossref] [PubMed]


