
Effect of prior thoracic radiotherapy on prognosis in relapsed small cell lung cancer patients treated with anlotinib: a subgroup analysis of the ALTER 1202 trial
Introduction
A total of 2,093,876 new cases of lung cancer and 1,761,007 related deaths were reported worldwide in 2018 (1). Most lung cancers are non-small cell lung cancer (NSCLC), but small cell lung cancer (SCLC) accounts for 13% to 15% of all lung cancers (2-4). SCLC is characterized by rapid doubling time, fast growth, and early metastatic spread (5). The annual incidence of SCLC has declined in industrialized countries over the past 30 years, likely due to the decreased rate of smoking in these regions (6,7), but the incidence of SCLC remains high in countries with prevalent smoking, as in China (8,9). Hence, despite the best management, the 5-year overall survival (OS) rate is less than 7%, and patients with extensive SCLC have a very poor prognosis (4,5). Thoracic radiotherapy (RT) is commonly used in the management of SCLC and is characterized by a limited duration of treatment, concurrent or consolidation therapy, and palliation of local symptoms (5). RT may cause tissue DNA damage and release of various inflammatory factors and fibrosis-related factors (10). Whether differences exist in the efficacy and safety of subsequent systemic therapies (such as chemotherapy, targeting, and immunity) for patients undergoing RT in the front line is worth exploring and analyzing.
Antiangiogenic therapies for SCLC include bevacizumab, thalidomide, vandetanib, sunitinib, Rh-endostatin, aflibercept, cediranib, nintedanib, apatinib, and anlotinib (11,12). Tyrosine kinase inhibitors (TKI) block signal transduction by the receptors, while antibodies bind either the receptors or their ligands, preventing their association (11,12). In addition, the different antiangiogenic therapies have different profiles of inhibition of the different receptors: vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and c-Kit. Angiogenesis is only one aspect of cancer growth besides uncontrolled cell growth, division, and dissemination (13,14). Different antiangiogenic drugs have different survival benefits (11,12). The original anlotinib ALTER 1202 study showed that both PFS and OS benefited from anlotinib monotherapy (15,16). Hence, combining therapies might target cancer cells from multiple fronts at the same time, achieving a better therapeutic effect. Still, the numbers of available drugs and possible permutations represent a bottleneck for research since the permutations have to be tried in order to determine the best ones in terms of efficacy, safety, and quality of life.
Anlotinib is a novel oral multitarget TKI that inhibits the VEGFR, PDGFR, FGFR, and c-Kit, thus inhibiting angiogenesis and tumor growth (17,18). It was approved by National Medical Products Administration (NMPA) for use against NSCLC in the ALTER0303 trial and against SCLC in the ALTER1202 trial (16,18). The ALTER 1202 trial showed that compared to placebo, the median PFS of patients with SCLC treated with anlotinib who had received at least second-line chemotherapy was extended by 3.4 months (4.1 vs. 0.7 months). The risk of disease progression or death was reduced by 81% [hazard ratio (HR) =0.19; 95% confidence interval (CI): 0.12–0.32; P<0.0001] (15,16). Tolerance and safety profiles were also favorable (15,16) and comparable to other studies (19-21).
In the ALTER 0303 trial, a phase III, double-blind trial in patients with NSCLC who received anlotinib as a third-line treatment, a subgroup analysis revealed that those who had received thoracic RT had a longer median PFS than did those who did not receive thoracic RT (22). Thoracic radiation therapy has been widely used for treating SCLC. Still, no data are available on the safety and efficacy of thoracic radiation therapy as a follow-up treatment to therapy with anlotinib.
Therefore, the innovation of the present study was to examine the impact of radiation therapy in patients with SCLC treated with anlotinib. This study was a subgroup analysis of the ALTER 1202 (which was a multicenter, randomized, double-blind, placebo-controlled phase 2 trial) trial performed to explore the effect of front-line RT on the benefits of subsequent anlotinib treatment. The results might help target patients who could benefit the most from anlotinib and allow tailored treatments against SCLC.
Methods
Study design and participants
This was a subgroup analysis of the ALTER 1202 trial (ClinicalTrial.gov; NCT03059797). The participants were patients with SCLC progression after at least 2 lines of chemotherapy. The eligible patients were randomized at a 2:1 ratio to receive anlotinib or placebo. The overall study design has been reported previously (15,16). The original trial was approved by the Ethical Committee of Jilin Cancer Hospital (approval number 201701-002-01) as the lead center and by the committees of all participating centers. The study was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice Guidelines. Written informed consent was obtained from each participant, including consent for the possibility of supplemental analyses.
Grouping
The patients were divided into previous thoracic RT and no previous thoracic RT (non-RT) subgroups. Each subgroup included anlotinib and placebo arms. The analysis of PFS and OS was then performed between anlotinib and placebo in different doses (<45 vs. ≥45 Gy) of radiation of the front-line chest RT.
Data collection
The data included the baseline characteristics of the participants, including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, smoking, SCLC stage, prior treatments, RT dose, and pattern of relapse, follow-up data (progression and death), and adverse events (AEs).
Outcomes
The outcomes included PFS, OS, objective response rate (ORR), disease control rate (DCR), and safety. PFS was defined as the time from randomization until objective progression or death from any cause according to response evaluation criteria in solid tumors (RECIST 1.1). OS was defined as the time between the start of enrollment and the time of death from any cause. ORR was defined as the percentage of patients who achieved a complete response (CR) or partial response (PR). DCR was defined as the percentage of patients with CR, PR, and stable disease over 4 weeks. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). All the definitions and assessments were the same as those used in the original ALTER 1202 trial (15,16).
Statistical analysis
All statistical analyses were performed using SAS 9.4 (SAS Institute, NY, USA). The baseline characteristics and AEs were summarized using descriptive statistics. PFS and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. The stratification factors in the ALTER 1202 trial (disease stage and pattern of relapse) were adjusted in the Cox proportional-hazards model. The ORR and DCR were compared between subgroups using Pearson’s chi-square test or Fisher’s exact test, as appropriate. All statistical tests were 2-sided, and P values <0.05 were considered statistically significant.
The PFS, OS, ORR, and DCR analyses were performed with the full analysis set, which included all the participants with at least 1 dose of the study drug according to the intention-to-treat principle. Safety analyses were performed using the safety analysis set, which included all participants who received at least 1 dose of the study drug and had records of safety.
Results
Participants
In the ALTER 1202 trial, 68 (57.1%) participants (anlotinib, n=46; placebo, n=22) received RT and 51 (42.9%) participants (anlotinib, n=35; placebo, n=16) did not. Table 1 presents the characteristics of the participants. The age of patients ranged from 48.0 to 66.5 years. There was a greater proportion of males, current smokers, and patients with extensive diseases. Most of the patients had good baseline performance status (ECOG score =1).
Table 1
| Variable | RT subgroup | Non-RT subgroup | |||
|---|---|---|---|---|---|
| Anlotinib (n=46) | Placebo (n=22) | Anlotinib (n=35) | Placebo (n=16) | ||
| Age (range), year | 55.0 (48.0–63.0) | 55.0 (51.0–63.0) | 57.0 (53.0–61.0) | 60.5 (52.0–66.5) | |
| Sex, n (%) | |||||
| Male | 31 (67.4) | 18 (81.8) | 24 (68.6) | 12 (75.0) | |
| Female | 15 (32.6) | 4 (18.2) | 11 (31.4) | 4 (25.0) | |
| ECOG performance status, n (%) | |||||
| 0 | 3 (6.5) | 1 (4.6) | 2 (5.7) | 1 (6.3) | |
| 1 | 41 (89.1) | 16 (72.7) | 31 (88.6) | 14 (87.5) | |
| 2 | 2 (4.4) | 5 (22.7) | 2 (5.7) | 1 (6.3) | |
| Smoking history, n (%) | |||||
| Never | 18 (39.1) | 7 (31.8) | 15 (42.9) | 4 (25.0) | |
| Current | 24 (52.2) | 15 (68.2) | 19 (54.3) | 12 (75.0) | |
| Former | 4 (8.7) | 0 | 1 (2.9) | 0 | |
| Disease stage, n (%) | |||||
| Limited stage | 9 (19.6) | 1 (4.5) | 0 | 3 (18.7) | |
| Extensive stage | 37 (80.4) | 21 (95.5) | 35 (100) | 13 (81.3) | |
| Previous lines of chemotherapy, n (%) | |||||
| 2 | 34 (73.9) | 15 (68.2) | 29 (82.9) | 13 (81.3) | |
| ≥3 | 12 (26.1) | 7 (31.8) | 6 (17.1) | 3 (18.7) | |
| Previous thoracic radiotherapy dose, n (%) | |||||
| <45 Gy | 7 (15.2) | 2 (9.1) | 0 | 0 | |
| ≥45 Gy | 28 (60.9) | 13 (59.1) | 0 | 0 | |
| Pattern of relapse, n (%) | |||||
| Sensitive | 11 (23.9) | 2 (9.1) | 3 (8.6) | 2 (12.5) | |
| Refractory | 35 (76.1) | 20 (90.9) | 32 (91.4) | 14 (87.5) | |
RT, radiotherapy; ECOG, Eastern Cooperative Oncology Group.
Progression-free survival (PFS)
As shown in Figure 1, the median PFS was 5.49 (2.83–6.47) months for anlotinib and 0.69 (0.66–0.76) months for placebo in the RT subgroup (P<0.0001). In the non-RT subgroup, the PFS was 2.83 (1.87–4.11) months for anlotinib and 0.76 (0.66–1.91) months for placebo (P=0.0003). In addition, PFS in the anlotinib arm was longer in the RT subgroup compared with the non-RT subgroup (P=0.0091, by log-rank test). In Cox analysis, with adjustments made for disease stage and pattern of relapse, the use of anlotinib was associated with PFS in the RT (HR 0.13; 95% CI: 0.06–0.28) and non-RT subgroups (HR 0.16; 95% CI: 0.07–0.38). In the anlotinib arm, when adjustments were made for disease stage and pattern of relapse, RT was associated with PFS (HR 0.50; 95% CI: 0.27–0.91). The PFS of anlotinib was longer in the RT subgroup (P=0.018).
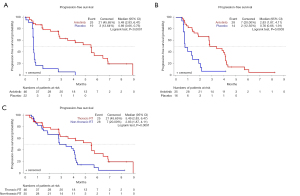
OS
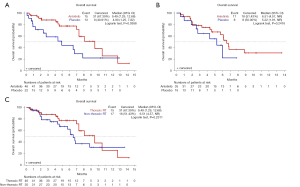
As shown in Figure 2, in the RT subgroup, median OS was 9.49 (95% CI: 7.29–12.68) months for anlotinib and 4.90 (95% CI: 1.25–7.82) months for placebo (P=0.0388 by log-rank test). In the non-RT subgroup, OS was 6.51 [95% CI: 4.57–NR (not reached)] months for anlotinib and 5.22 (95% CI: 1.91–NR) months for placebo (P=0.2416 by log-rank test). No significant difference in OS was found between the RT and non-RT subgroups in the anlotinib arm (P=0.2311, by log-rank test). In Cox analysis, when adjustments were made for disease stage and pattern of relapse, the use of anlotinib was associated with OS in the RT subgroup (HR 0.47; 95% CI: 0.22–0.98) and not obviously associated with OS in the non-RT subgroup (HR 0.54; 95% CI: 0.22–1.33). In the anlotinib arm, when adjustments were made for disease stage and pattern of relapse, RT was not obviously associated with OS (HR 0.68; 95% CI: 0.32–1.45). There was no significant difference associated with anlotinib treatment (P=0.587). The PFS and OS between patients in the RT group who received <45 or ≥45 Gy of RT dose were similar (Table S1).
Objective response and DCRs
Table 2 presents the tumor responses. No significant differences in the ORR were found between the RT (P=0.454) and non-RT (P=0.9999) subgroups in the two arms. The DCR was higher in the anlotinib arm of the RT subgroup compared with that of the placebo arm (73.9% vs. 9.1%; P<0.001) and the non-RT subgroup (68.6% vs. 18.8%; P=0.002).
Table 2
| RT subgroup | Non-RT subgroup | P for interaction | ||||||
|---|---|---|---|---|---|---|---|---|
| Anlotinib (n=46) | Placebo (n=22) | P | Anlotinib (n=35) | Placebo (n=16) | P | |||
| Complete response, n (%) | 0 | 0 | – | 0 | 0 | |||
| Partial response, n (%) | 2 (4.4) | 0 | 2 (5.7) | 1 (6.3) | ||||
| Stable disease, n (%) | 32 (69.6) | 2 (9.1) | 22 (62.9) | 2 (12.5) | ||||
| Progression disease, n (%) | 10 (21.7) | 14 (63.6) | 10 (28.6) | 11 (68.8) | ||||
| Nonevaluable, n (%) | 2 (4.4) | 6 (27.3) | 1 (2.9) | 2 (12.5) | ||||
| Objective response rate, n (%) | 2 (4.4) | 0 | 0.454 | 2 (5.7) | 1 (6.3) | 0.9999 | ||
| 95% CI | (0.5, 14.8) | – | (0.0, 20.0) | (0.0, 30.0) | ||||
| Disease control rate, n (%) | 34 (73.9) | 2 (9.1) | <0.001 | 24 (68.6) | 3 (18.8) | 0.002 | ||
| 95% CI | (58.9, 85.7) | (1.1, 29.2) | (50.7, 83.2) | (5.8, 43.8) | ||||
| Median progression-free survival, (month) | 5.49 | 0.69 | <0.0001 | 2.83 | 0.76 | 0.0003 | 0.018 | |
| 95% CI | (2.83, 6.47) | (0.66, 0.76) | (1.87, 4.11) | (0.66, 1.91) | ||||
| Median overall survival, (month) | 9.49 | 4.90 | 0.0388 | 6.51 | 5.22 | 0.242 | 0.587 | |
| 95% CI | (7.29, 12.68) | (1.25, 7.82) | (4.57, –) | (1.91, –) | ||||
CI, confidence interval; RT, radiotherapy.
Safety
In regard to safety, 1 (2.2%) and 2 (9.1%) deaths were reported for anlotinib and placebo in the RT subgroups, and 3 (8.6%) and 1 (6.3%) death for placebo in the non-RT subgroups. Table 3 presents the AEs in the RT subgroup. The rates of AEs and serious AEs (SAEs) were similar between the two arms of the RT subgroup; 4 (8.7%) participants in the anlotinib arm had grade >3 AEs leading to dose reduction, while no patients in the placebo arm experienced grade 3 AEs; 6 (13.0%) participants in the anlotinib arm had grade >3 AEs leading to treatment discontinuation versus 2 (9.1%) in the placebo arm; 1 (2.2%) patient had an AE leading to death in the anlotinib arm compared with 2 (9.1%) in the placebo arm. The main AEs in the anlotinib arm were hypertension, weight loss, hypertriglyceridemia, leukopenia, hypercholesterolemia, and fatigue.
Table 3
| Anlotinib (n=46) | Placebo (n=22) | ||||
|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | ||
| AE | 46 (100.0) | 25 (54.3) | 22 (100.0) | 11 (50.0) | |
| SAE | 13 (28.3) | 10 (21.7) | 7 (31.8) | 6 (27.3) | |
| AE leading to dose reduction | 4 (8.7) | 4 (8.7) | 0 | 0 | |
| AE leading to termination of treatment | 6 (13.0) | 6 (13.0) | 3 (13.6) | 2 (9.1) | |
| AE leading to death | 1 (2.2) | 2 (9.1) | |||
| AEs in ≥10% (any grade) or ≥2% (grade ≥3) of patients in either arm | |||||
| Hypertension | 18 (39.1) | 7 (15.2) | 0 | 0 | |
| Weight loss | 18 (39.1) | 0 | 2 (9.1) | 0 | |
| Hypertriglyceridemia | 17 (37.0) | 5 (10.9) | 0 | 0 | |
| Leukopenia | 14 (30.4) | 0 | 3 (13.6) | 0 | |
| Hypercholesterolemia | 12 (26.1) | 2 (4.4) | 2 (9.1) | 0 | |
| Fatigue | 12 (26.1) | 0 | 8 (36.4) | 0 | |
| Loss of appetite | 11 (23.9) | 0 | 5 (22.7) | 0 | |
| Elevated γ-glutamyltransferase level | 10 (21.7) | 4 (8.7) | 5 (22.7) | 1 (4.6) | |
| Expectoration | 10 (21.7) | 2 (4.4) | 3 (13.6) | 0 | |
| Backache | 9 (19.6) | 0 | 0 | 0 | |
| Elevated alanine aminotransferase level | 9 (19.6) | 0 | 3 (13.6) | 2 (9.1) | |
| Elevated aspartate aminotransferase level | 9 (19.6) | 3 (6.5) | 3 (13.6) | 0 | |
| Palmoplantar redness syndrome | 9 (19.6) | 3 (6.5) | 0 | 0 | |
| Hypothyroidism | 8 (17.4) | 0 | 0 | 0 | |
| Cough | 8 (17.4) | 0 | 2 (9.1) | 0 | |
| Decreased lymphocyte count | 8 (17.4) | 3 (6.5) | 4 (18.2) | 3 (13.6) | |
| Urine red blood cell positive | 8 (17.4) | 0 | 0 | 0 | |
| Increased blood thyroid-stimulating hormone level | 8 (17.4) | 0 | 0 | 0 | |
| Proteinuria | 7 (15.2) | 0 | 0 | 0 | |
| Difficulty in pronunciation | 7 (15.2) | 0 | 0 | 0 | |
| Difficulty breathing | 7 (15.2) | 2 (4.4) | 3 (13.6) | 1 (4.6) | |
| ECG QT interval extension | 7 (15.2) | 0 | 3 (13.6) | 0 | |
| Reduced neutrophil count | 7 (15.2) | 0 | 3 (13.6) | 0 | |
| Sinus tachycardia | 7 (15.2) | 1 (2.2) | 5 (22.7) | 0 | |
| Gum pain | 6 (13.0) | 0 | 0 | 0 | |
| Nausea | 6 (13.0) | 1 (2.2) | 3 (13.6) | 0 | |
| Diarrhea | 6 (13.0) | 0 | 2 (9.1) | 0 | |
| Hyperglycemia | 6 (13.0) | 1 (2.2) | 4 (18.2) | 0 | |
| Urine leukocyte positive | 6 (13.0) | 0 | 0 | 0 | |
| Vomiting | 6 (13.0) | 1 (2.2) | 5 (22.7) | 1 (4.6) | |
| Increased blood alkaline phosphatase level | 6 (13.0) | 0 | 3 (13.6) | 0 | |
| Fever | 5 (10.9) | 0 | 2 (9.1) | 0 | |
| Stomachache | 5 (10.9) | 0 | 0 | 0 | |
| Elevated bilirubin level | 5 (10.9) | 2 (4.4) | 0 | 0 | |
| Hemoptysis | 5 (10.9) | 1 (2.2) | 2 (9.1) | 1 (4.6) | |
| Anemia | 5 (10.9) | 0 | 6 (27.3) | 1 (4.6) | |
| Weight gain | 5 (10.9) | 0 | 0 | 0 | |
| Dizziness | 5 (10.9) | 0 | 3 (13.6) | 0 | |
| Chest pain | 5 (10.9) | 0 | 4 (18.2) | 0 | |
| Elevated blood bilirubin level | 5 (10.9) | 1 (2.2) | 0 | 0 | |
| Limb pain | 5 (10.9) | 0 | 0 | 0 | |
| Hyponatremia | 4 (8.7) | 1 (2.2) | 4 (18.2) | 3 (13.6) | |
| Decreased platelet count | 4 (8.7) | 1 (2.2) | 3 (13.6) | 2 (9.1) | |
| Hypophosphatemia | 3 (6.5) | 1 (2.2) | 0 | 0 | |
| Skeletal muscle pain | 3 (6.5) | 1 (2.2) | 0 | 0 | |
| Hard to swallow | 3 (6.5) | 1 (2.2) | 0 | 0 | |
AE, adverse event; ECG, electrocardiogram; SAE, serious adverse event.
Table 4 presents the AEs in the non-RT subgroup. As in the RT subgroup, the rates of AEs and SAEs were similar between the two arms of the RT subgroup; 1 (2.9%) participant had grade >3 AEs leading to dose reduction in the anlotinib arm versus none in the placebo arm; 2 (5.7%) participants in the anlotinib arm had grade >3 AEs leading to treatment discontinuation versus 1 (6.3%) in the placebo arm; 3 (8.6%) participants experienced an AE leading to death in the anlotinib arm compared with 1 (6.3%) in the placebo arm. The main AEs in the anlotinib arm were hypertension, loss of appetite, hyponatremia, sinus tachycardia, anemia, fatigue, leukopenia, weight loss, and elevated γ-glutamyltransferase.
Table 4
| Anlotinib (n=35) | Placebo (n=16) | ||||
|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | ||
| AE | 35 (100.0) | 18 (51.4) | 16 (100.0) | 5 (31.3) | |
| SAE | 9 (25.7) | 8 (22.9) | 3 (18.8) | 3 (18.8) | |
| AE leading to dose reduction | 1 (2.9) | 1 (2.9) | 0 | 0 | |
| AE leading to termination of treatment | 5 (14.3) | 2 (5.7) | 1 (6.3) | 1 (6.3) | |
| AE leading to death | 3 (8.6) | 1 (6.3) | |||
| AEs in ≥10% (any grade) or ≥2% (grade ≥3) of patients in either arm | |||||
| Hypertension | 16 (45.7) | 4 (11.4) | 1 (6.3) | 0 | |
| Loss of appetite | 14 (40.0) | 1 (2.9) | 5 (31.3) | 1 (6.3) | |
| Hyponatremia | 13 (37.1) | 7 (20.0) | 3 (18.8) | 1 (6.3) | |
| Sinus tachycardia | 13 (37.1) | 0 | 1 (6.3) | 0 | |
| Anemia | 12 (34.3) | 0 | 4 (25.0) | 1 (6.3) | |
| Fatigue | 11 (31.4) | 1 (2.9) | 5 (31.3) | 1 (6.3) | |
| Leukopenia | 10 (28.6) | 2 (5.7) | 2 (12.5) | 0 | |
| Weight loss | 10 (28.6) | 0 | 0 | 0 | |
| Elevated γ-glutamyltransferase level | 9 (25.7) | 2 (5.7) | 4 (25.0) | 3 (18.8) | |
| Hyperglycemia | 8 (22.9) | 0 | 2 (12.5) | 0 | |
| Increased blood thyroid-stimulating hormone level | 8 (22.9) | 0 | 0 | 0 | |
| Palmoplantar redness syndrome | 8 (22.9) | 1 (2.9) | 0 | 0 | |
| Elevated alanine aminotransferase level | 7 (20.0) | 1 (2.9) | 3 (18.8) | 0 | |
| Diarrhea | 7 (20.0) | 0 | 0 | 0 | |
| Hypercholesterolemia | 7 (20.0) | 0 | 1 (6.3) | 0 | |
| Hypertriglyceridemia | 7 (20.0) | 1 (2.9) | 0 | 0 | |
| Elevated aspartate aminotransferase level | 7 (20.0) | 0 | 3 (18.8) | 0 | |
| ECG QT interval extension | 7 (20.0) | 0 | 3 (18.8) | 1 (6.3) | |
| Decreased platelet count | 7 (20.0) | 1 (2.9) | 2 (12.5) | 0 | |
| Hypoalbuminemia | 6 (17.1) | 0 | 3 (18.8) | 0 | |
| Elevated bilirubin level | 6 (17.1) | 2 (5.7) | 0 | 0 | |
| Cough | 6 (17.1) | 0 | 1 (6.3) | 0 | |
| Chest pain | 6 (17.1) | 0 | 1 (6.3) | 0 | |
| Decreased white blood cell count | 5 (14.3) | 0 | 0 | 0 | |
| Proteinuria | 5 (14.3) | 0 | 0 | 0 | |
| Hypochloremia | 5 (14.3) | 1 (2.9) | 2 (12.5) | 0 | |
| Difficulty breathing | 5 (14.3) | 1 (2.9) | 0 | 0 | |
| Hypothyroidism | 5 (14.3) | 0 | 1 (6.3) | 0 | |
| Oropharyngeal pain | 5 (14.3) | 0 | 0 | 0 | |
| Elevated blood bilirubin level | 5 (14.3) | 1 (2.9) | 1 (6.3) | 0 | |
| Limb pain | 5 (14.3) | 0 | 0 | 0 | |
| Increased lipase level | 5 (14.3) | 4 (11.4) | 0 | 0 | |
| Constipation | 4 (11.4) | 0 | 1 (6.3) | 0 | |
| Hypophosphatemia | 4 (11.4) | 4 (11.4) | 0 | 0 | |
| Nausea | 4 (11.4) | 0 | 1 (6.3) | 0 | |
| Expectoration | 4 (11.4) | 0 | 1 (6.3) | 0 | |
| Urine red blood cell positive | 4 (11.4) | 0 | 0 | 0 | |
| Elevated amylase | 3 (8.6) | 2 (5.7) | 0 | 0 | |
| Lung infection | 3 (8.6) | 1 (2.9) | 0 | 0 | |
| Vomiting | 3 (8.6) | 0 | 2 (12.5) | 0 | |
| Death | 3 (8.6) | 3 (8.6) | 1 (6.3) | 1 (6.3) | |
| Increased blood alkaline phosphatase level | 3 (8.6) | 0 | 3 (18.8) | 0 | |
| Reduced neutrophil count | 3 (8.6) | 1 (2.9) | 0 | 0 | |
| Hypoglycemia | 1 (2.9) | 1 (2.9) | 0 | 0 | |
| Bone marrow failure | 1 (2.9) | 1 (2.9) | 0 | 0 | |
| Intravenous stent implantation | 1 (2.9) | 1 (2.9) | 0 | 0 | |
| Rash | 1 (2.9) | 1 (2.9) | 0 | 0 | |
| Hyperuricemia | 0 | 0 | 2 (12.5) | 0 | |
| Gastroesophageal reflux disease | 0 | 0 | 2 (12.5) | 0 | |
AE, adverse event; ECG, electrocardiogram; SAE, serious adverse event.
Discussion
The ALTER 1202 trial showed that anlotinib prolonged the PFS and OS of patients with SCLC as a third-line treatment or above (15,16), but the impact of previous thoracic RT was unclear. Therefore, this study aimed to explore the effect of front-line RT on the benefits of anlotinib. The results suggested that compared with the placebo arm, the patients who received anlotinib had PFS benefits in the front-line RT and non-RT subgroups. PFS in the anlotinib arm improved in the RT subgroup compared with the non-RT subgroup. Despite the wealth of data in the literature about the effect of radiation therapy in patients with SCLC, this study innovated by examining the impact of radiation therapy in patients with SCLC treated with anlotinib.
Anlotinib is an antiangiogenic TKI that affects the tumor microenvironment (23,24). Previous studies have suggested that anlotinib is likely to be effective against lung cancer (15-18,23,25,26). Nevertheless, the comparison of the results of the present study with the published findings was limited by the fact that no similar study comparing the prognosis of antiangiogenic drugs versus placebo in patients with or without front-line RT has been conducted to date. Only one study explored the effect of front-line RT versus no RT on the prognosis of rear-line antiangiogenic treatments (22). The study included patients with NSCLC treated with mediastinal RT, and the analysis was made in the anlotinib cohort. The patients treated with anlotinib in the chest RT subgroup had a longer median PFS compared with patients in the non-RT subgroup (5.93 vs. 4.63 months; P=0.027) with an HR of 0.68 (95% CI: 0.52–0.96), but no significant differences in OS were noted (22). In the present study, the patients in the anlotinib arm who received front-line RT had better PFS compared with patients in the non-RT subgroup. The difference between the RT and non-RT subgroups was more pronounced than in the previous study (22) (5.49 vs. 2.83 months, compared with 5.93 vs. 4.63 months); some benefit in terms of OS was observed, but it was not statistically significant. In addition, the present subgroup analysis showed that, as long as front-line chest RT has been performed, a PFS benefit can be achieved with anlotinib regardless of the RT dose. The results agreed with the findings of the only study that explored the effect of front-line RT versus no RT (22). However, the discrepancy in the difference in PFS might be due to the large difference in epidemiological characteristics between SCLC and NSCLC, which might have influenced the results. In addition, the sample sizes were small, the RT regimens were not uniform, and the different lines of treatment before anlotinib could have differed. Nevertheless, these results on anlotinib were promising because no previous study on the use of TKIs as third-line therapy against lung cancer has found varied benefits (27-30).
Of note, the results suggested that RT, as a local treatment, improved the subsequent response to anlotinib. Non-RT patients only achieved a PFS benefit, while RT patients achieved both OS and PFS benefits. RT has been shown to play an important role in the induction of an immune response against the tumor (31). Indeed, RT can increase the expression of antigens, the release of pro-inflammatory cytokines, increase the recruitment of cytotoxic immune cells, and promote antigen presentation (32). Since anti-VEGF therapy also induces an immune permissive status (33), the combination of RT and anlotinib might be synergistic in activating antitumor immunity. Studies are still necessary to identify the mechanisms.
Concurrent chemoradiotherapy can be used in patients with limited-stage SCLC (2,5,34). The chemotherapy regimen of cisplatin combined with etoposide for 4–6 cycles is recommended, and chest RT involved can be used from the first or second cycle (5,35). The RT regimen can be hyperfractionation RT (1.5 Gy/time, twice a day, total dose of 45 Gy) or the conventional fractionation dose of a higher dose (1.8–2.0 Gy/time, total dose of 60 Gy) (5,35,36). With this combination regimen, a CR rate of >80% was achieved, and median survival and 5-year tumor-free survival were improved (5,35-37). Patients with extensive SCLC receive chemotherapy in priority to control the metastatic spread first. Then, chest RT is recommended for the patients who achieved CR or PR after chemotherapy to control the local disease. Still, few studies examined chest RT, and no study examined the radiation dose specifically. Some studies recommend a short course and slightly higher fractionation RT. For example, the CREST randomized controlled study used 30 Gy over 10 fractions (38), and the Canadian study used 40 Gy over 15 times (39). In the cancer hospitals affiliated to the Chinese Academy of Sciences, most patients receive conventional fractionation RT with high safety. The dose analysis was conducted according to the standardization of the LQ model. The results suggest that increasing the dose of chest RT within a certain range can improve the PFS and OS rates of the patients, indicating that for some patients with extensive-stage SCLC, more aggressive chest RT has a positive effect on delaying disease progression and improving survival. Still, there is no specific study on the delineation of the target areas for extensive SCLC, and most centers follow the principle of target areas for localized SCLC. The gross tumor volume (GTV) is delineated according to the lesions shown in imaging data after chemotherapy, and the clinical tumor volume (CTV) is delineated by referring to the scope of lymph node invasion before chemotherapy, without preventive lymph node irradiation (40).
In the present study, the safety profile was tolerable and similar to that in other studies on anlotinib (19) and the original ALTER 1202 trial (15,16). The safety of the antiangiogenic drug anlotinib was tolerable for patients with front-line RT and no RT. No obvious differences were observed between the two subgroups, suggesting that prior exposure to RT did not lead to new safety signals. Still, some minute differences were observed in the AEs among the subgroups, but the relationship of RT with these AEs is unknown and cannot be inferred from the present study.
This study had some limitations. First, it was a subgroup analysis based on a previous trial. Moreover, it had a small sample size and likely lacked sufficient power to detect an OS difference in the non-RT cohort. The analysis was limited to the data available in the original trial. For example, the rate of development of symptomatic chest failures in extensive-stage SCLC after undergoing post-chemotherapy chest RT was not documented. Finally, the sample size was too small to allow for analyses according to mutations or the presence of brain metastases. Indeed, different mutations in SCLC affect the outcomes, and brain metastasis is a factor of poor prognosis (5). Nevertheless, the study provided preliminary data that could be used to plan future studies.
Immunotherapy plays an increasingly important part in the personalized management of SCLC (41,42). Previous studies examined the possible combination of immunotherapy and RT in SCLC, with promising results (43). Another study showed that the combination of anlotinib with PD-1 blockade shows some promising efficacy and manageable AEs (44). The IMpower133 (45) and CASPIAN (46) trials showed that atezolizumab or durvalumab combined with chemotherapy prolonged the OS for 2 months, and they were approved for the therapy for SCLC. Still, due to the relatively limited benefits of OS, how to improve the efficacy of immunotherapy in SCLC is an important direction of exploration at present. In this way, penpulimab combined with anlotinib showed favorable antitumor activity in patients with SCLC and failure to platinum-based regimens (47). Another trial suggested that toripalimab with anlotinib and chemotherapy was effective in patients with treatment-naïve extensive-stage SCLC (48).
Conclusions
Anlotinib can be used as a third-line and above therapy against SCLC. It benefits patients with or without front-line RT, and its safety profile is tolerable. Front-line RT has an impact on subsequent PFS when using anlotinib.
Acknowledgments
We thank all the participating patients and their families, study personnel at all sites, and the ALTER 1202 clinical trial team. The authors also appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: The study was supported by the Special Project for Significant New Drug Research and Development in the Major National Science and Technology Projects of China (project No. 2020ZX09201-024); the Provincial Health and Family Planning Commission (grant number 2019J077); Province Development and Reform Commission (grant numbers 2021C043-1, 2021C042-7); Science and Technology Agency of Jilin Provincial Project (grant number 20200201518JC); and Chia-tai Tianqing Pharmaceutical Group Co., Ltd. (provided the study drug). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnote
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tlcr-21-632
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-632). HSP receives research grant from U.S. FDA, consulting fees from AstraZeneca, Guidepoint and Grand Rounds Health, payments from Bristol Myers Squibb, Varian Medical Systems, Rad Onc Questions, and support for attending meeting from Varian Medical Systems. All authors received support from Chia-tai Tianqing Pharmaceutical Group Co., Ltd. (provided the study drug). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice Guidelines. The original trial was approved by the Ethical Committee of Jilin Cancer Hospital (approval number 201701-002-01) as the lead center and by the committees of all participating centers. Written informed consent was obtained from each participant, including consent for the possibility of supplemental analyses.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ost DE, Jim Yeung SC, Tanoue LT, et al. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e121S-e141S.
- Higgins KA, Gorgens S, Sudmeier LJ, et al. Recent developments in limited stage small cell lung cancer. Transl Lung Cancer Res 2019;8:S147-52. [Crossref] [PubMed]
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664-72. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Small Cell Lung Cancer. Version 3.2021. National Comprehensive Cancer Network; 2021.
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
- Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health 2019;85:8. [Crossref] [PubMed]
- Liu J, Cheng Y, Li H, et al. Current Status of Small Cell Lung Cancer in China. J Cancer Biol Res 2014;2:1032.
- Shi Y, Xing P, Fan Y, et al. Current small cell lung cancer treatment in China. Thorac Cancer 2015;6:233-8. [Crossref] [PubMed]
- Giuranno L, Ient J, De Ruysscher D, et al. Radiation-Induced Lung Injury (RILI). Front Oncol 2019;9:877. [Crossref] [PubMed]
- Li Q, Wu T, Jing L, et al. Angiogenesis inhibitors for the treatment of small cell lung cancer (SCLC): A meta-analysis of 7 randomized controlled trials. Medicine (Baltimore) 2017;96:e6412 [Crossref] [PubMed]
- Montanino A, Manzo A, Carillio G, et al. Angiogenesis Inhibitors in Small Cell Lung Cancer. Front Oncol 2021;11:655316 [Crossref] [PubMed]
- Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer 2021;21:162-80. [Crossref] [PubMed]
- Chaudhuri PK, Low BC, Lim CT. Mechanobiology of Tumor Growth. Chem Rev 2018;118:6499-515. [Crossref] [PubMed]
- Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol 2019;12:47. [Crossref] [PubMed]
- Cheng Y, Wang Q, Li K, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled Phase 2 study. Br J Cancer 2021;125:366-71. [Crossref] [PubMed]
- Wang L, He Z, Yang S, et al. The impact of previous therapy strategy on the efficiency of anlotinib hydrochloride as a third-line treatment on patients with advanced non-small cell lung cancer (NSCLC): a subgroup analysis of ALTER0303 trial. Transl Lung Cancer Res 2019;8:575-83. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Zhang K, Ma X, Gao H, et al. Efficacy and Safety of Anlotinib in Advanced Non-Small Cell Lung Cancer: A Real-World Study. Cancer Manag Res 2020;12:3409-17. [Crossref] [PubMed]
- Zhong Q, Liu Z. Efficacy and Safety of Anlotinib in Patients with Advanced Non-Small Cell Lung Cancer: A Real-World Study. Cancer Manag Res 2021;13:4115-28. [Crossref] [PubMed]
- Zhai C, Zhang X, Ren L, et al. The Efficacy and Safety of Anlotinib Combined With PD-1 Antibody for Third-Line or Further-Line Treatment of Patients With Advanced Non-Small-Cell Lung Cancer. Front Oncol 2021;10:619010 [Crossref] [PubMed]
- Wang L, He Z, Yang S, et al. The impact of previous therapy strategy on the efficiency of anlotinib hydrochloride as a third-line treatment on patients with advanced non-small cell lung cancer (NSCLC): a subgroup analysis of ALTER0303 trial. Transl Lung Cancer Res 2019;8:575-83. [Crossref] [PubMed]
- Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. [Crossref] [PubMed]
- Liu S, Qin T, Liu Z, et al. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis 2020;11:309. [Crossref] [PubMed]
- Chen XZ. Anlotinib for Refractory Advanced Non-Small Cell Lung Cancer in China. JAMA Oncol 2019;5:116-7. [Crossref] [PubMed]
- Shao L, Wang W, Song Z, et al. The efficacy and safety of anlotinib treatment for advanced lung cancer. Onco Targets Ther 2019;12:6549-54. [Crossref] [PubMed]
- Paz-Ares L, Hirsh V, Zhang L, et al. Monotherapy Administration of Sorafenib in Patients With Non-Small Cell Lung Cancer (MISSION) Trial: A Phase III, Multicenter, Placebo-Controlled Trial of Sorafenib in Patients with Relapsed or Refractory Predominantly Nonsquamous Non-Small-Cell Lung Cancer after 2 or 3 Previous Treatment Regimens. J Thorac Oncol 2015;10:1745-53. [Crossref] [PubMed]
- Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol 2012;30:2070-8. [Crossref] [PubMed]
- Weiss JM, Villaruz LC, Socinski MA, et al. A single-arm phase II trial of pazopanib in patients with advanced non-small cell lung cancer with non-squamous histology with disease progression on bevacizumab containing therapy. Lung Cancer 2014;86:288-90. [Crossref] [PubMed]
- Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:1059-66. [Crossref] [PubMed]
- Teng F, Kong L, Meng X, et al. Radiotherapy combined with immune checkpoint blockade immunotherapy: Achievements and challenges. Cancer Lett 2015;365:23-9. [Crossref] [PubMed]
- Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756-63. [Crossref] [PubMed]
- Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol 2018;52:117-24. [Crossref] [PubMed]
- Gridelli C, Casaluce F, Sgambato A, et al. Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that's the question. Transl Lung Cancer Res 2016;5:150-4. [Crossref] [PubMed]
- Albain KS, Crowley JJ, Turrisi AT 3rd, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol 2002;20:3454-60. [Crossref] [PubMed]
- Chen GY, Jiang GL, Wang LJ, et al. Cisplatin/etoposide chemotherapy combined with twice daily thoracic radiotherapy for limited small-cell lung cancer: a clinical phase II trial. Int J Radiat Oncol Biol Phys 2005;61:70-5. [Crossref] [PubMed]
- Levy A, Botticella A, Le Péchoux C, et al. Thoracic radiotherapy in small cell lung cancer-a narrative review. Transl Lung Cancer Res 2021;10:2059-70. [Crossref] [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [Crossref] [PubMed]
- Yee D, Butts C, Reiman A, et al. Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiother Oncol 2012;102:234-8. [Crossref] [PubMed]
- Couñago F, de la Pinta C, Gonzalo S, et al. GOECP/SEOR radiotherapy guidelines for small-cell lung cancer. World J Clin Oncol 2021;12:115-43. [Crossref] [PubMed]
- Esposito G, Palumbo G, Carillio G, et al. Immunotherapy in Small Cell Lung Cancer. Cancers (Basel) 2020;12:2522. [Crossref] [PubMed]
- Konala VM, Madhira BR, Ashraf S, et al. Use of Immunotherapy in Extensive-Stage Small Cell Lung Cancer. Oncology 2020;98:749-54. [Crossref] [PubMed]
- Lin AJ, Roach M, Bradley J, et al. Combining stereotactic body radiation therapy with immunotherapy: current data and future directions. Transl Lung Cancer Res 2019;8:107-15. [Crossref] [PubMed]
- Zhang X, Zeng L, Li Y, et al. Anlotinib combined with PD-1 blockade for the treatment of lung cancer: a real-world retrospective study in China. Cancer Immunol Immunother 2021;70:2517-28. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Zhang C, Yang S, Chen J, et al. Penpulimab plus anlotinib as second-line treatment for the small cell lung cancer after failure of platinum-based systemic chemotherapy. J Clin Oncol 2021;39:8568. [Crossref]
- Luo H, Jian D, Feng Y, et al. Efficacy and safety of toripalimab with anlotinib and chemotherapy as first-line therapy in patients with extensive-stage small-cell lung cancer: Preliminary results of an open-label, single-arm, phase II study. J Clin Oncol 2021;39:e20570 [Crossref]


