
Cyclin-dependent kinase 4 upregulation mediates acquired resistance of dabrafenib plus trametinib in BRAF V600E-mutated lung cancer
Introduction
Since the discovery of the oncogenic mutation in the B-Raf proto-oncogene, serine/threonine kinase V600E (BRAF V600E), combination therapy with the B-Raf inhibitor, dabrafenib, and the MEK inhibitor, trametinib (DT) has been used for treating BRAF V600E-mutated non-small cell lung cancer (NSCLC) (1). Although mutations in the mitogen-activated protein kinase (MAPK) or phosphatidylinositol 3-kinase (PI3K)/AKT pathways have been reported, the mechanisms of resistance to DT therapy are unclear (2-4).
Cyclin-dependent kinase 4 (CDK4) is involved in regulating cell proliferation during the G1 phase. CDK4 forms a complex with cyclin D, which is regulated by MAPK activity, thereby mediating retinoblastoma (Rb) phosphorylation and resulting in cell cycle progression (5). CDK4/6 inhibitors have been used as anticancer agents in hormone receptor-positive breast cancer and KRAS-mutated NSCLC (6). Moreover, combined inhibition of RAF and CDK4/6 may have synergistic effects on tumors with RAS or RAF mutations (7). Additionally, CD4/6 inhibitors can overcome resistance to BRAF inhibitors caused by cyclin D1 upregulation in BRAF V600E-mutated melanoma (8). Thus, targeting CDK may overcome resistance to RAS- and RAF-mutant cancers. CDK4 may be implicated in the development of resistance in BRAF V600E-mutant NSCLC; however, the involvement of CDK4 in acquired resistance is unclear.
Here, we established clinically resistant cell lines derived from pleural effusion samples of patients with NSCLC. We report two cases of clinically DT-resistant BRAF V600E-mutated NSCLC and compared the expression levels of cell cycle-related genes, including CDK4, in DT-naïve and DT-resistant cases. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-415).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All study participants provided informed consent and this study was approved by the Institutional Review Board of Asahikawa Medical University (No.: 18185/19081). Written consent was obtained from all patients whose samples were analyzed, including pleural effusions.
Patients and treatments
Four patients were treated with DT, two of whom developed carcinomatous pleuritis after treatment. Pleural fluid from these two patients (LC-1, LC-6) was used for post-DT samples; lung tissue from corresponding patients collected before DT treatment was used for DT-naïve samples. The BRAF V600E mutant (IG: pleural effusion; ON: transtracheal lymph node biopsy) and the BRAF K601E mutant (MY: pleural effusion) were also subjected to the Oncomine Dx target test (Thermo Fisher Scientific, Waltham, MA, USA), and cancer cells derived from DT-naïve patients harboring these genetic mutations were used as a reference.
Establishment of cell line and expression constructs
Lymph node biopsies of patients with the BRAF_V600E mutation (ON) were performed, and the excess specimens were used. The isolated lung cancer cells were cultured in RPMI 1640 medium (GIBCO, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS, 100 units/mL penicillin, 100 mg/mL streptomycin and 1 mmol/L sodium pyruvate as the primary culture medium. After confirming that the tumor cells were proliferating, single cells were cultured in 96-well plates, and only the proliferating lung cancer cells were passaged as cell lines.
The CDK4 cDNA vector was a gift from Sander van den Heuvel (Addgene, plasmid #1876, Watertown, MA, USA). The CDK4 cDNA vector or empty vector was transfected by electrophoresis (Neon system, Thermo Fisher Scientific, Waltham, MA, USA). Dissociated ON cells were resuspended in 8 µL of Neon Resuspension Buffer R for every one million cells. For each electroporation, cells and 2 µL of vector were aliquoted into a sterile microcentrifuge tube. A Neon Tip was inserted into the Neon Pipette and the cell-DNA mixture was aspirated into the tip avoiding air bubbles. The Neon Pipette was then inserted into the Neon Tube containing 3 mL of Neon Electrolytic Buffer E in the Neon Pipette Station. Cells were pulsed twice with a voltage of 1,400 and a width of 20. After the pulse, cells were quickly transferred into a RPMI1640 medium without antibiotics.
Cell proliferation and growth assays
Dabrafenib and erlotinib were purchased from Selleckchem (Boston, MA, USA) and inhibition of growth was assessed by MTS assay according to previously established methods (9). All experimental points were set up in 6 to 12 wells and all experiments were repeated 2 times.
Genetic analysis
BRAF mutations were identified by the droplet digital polymerase chain reaction (PCR) or OncomineDx Target Test (Thermo Fisher Scientific, Waltham, MA, USA) in 12 of 233 patients with NSCLC over a 5-year period (2015–2020) in Asahikawa Medical University Hospital. DNA was extracted from bronchial lavage fluid (QIAamp DNA Mini Kit, QIAGEN, Hilden, Germany). Digital PCR was performed using a BRAF V600E-specific probe (Taqman cfDNA assay BRAF V600E, A44177 Thermo Fisher Scientific, Waltham, MA, USA) according to the QuantStudio3D (Thermo Fisher Scientific, Waltham, MA, USA) protocol. Genomic DNA mutations and copy number variations (CNVs) were assessed by a comprehensive cancer panel (160 genes, GeneRead DNAseq Targeted HC panel v2; QIAGEN, Hilden, Germany) on an Illumina MiSeq platform (Appendix 1).
mRNA expression analysis
mRNA was extracted from formalin-fixed paraffin-embedded samples of LC-1 and LC-6 samples using RNeasy Mini Kit (QIAGEN, Hilden, Germany). mRNA expression was assessed by the real-time PCR using a TaqMan Array human anticancer drug panel (RAPRJ2Y, Thermo Fisher Scientific, Waltham, MA, USA).
Antibodies and Western blotting
Cell lysis, Western blotting and immunohistochemistry (IHC) was done as previously described (9,10). The following antibodies were used for immunostaining and Western blotting: anti-CDK4 (D9G3E), anti-cyclin D1 (92G2) and anti-β-actin (13E5) (Cell Signaling Technology, Danvers, MA, USA). The receptor tyrosine kinase (RTK) array (Proteome Profiler Human Phospho-RTK Array Kit, R&D Systems, Minneapolis, MN, USA) was purchased and used according to the manufacturer’s recommended conditions as previously described (9). List of 49 RTKs measuring relative phosphorylation status on antibody arrays were shown in Appendix 2.
Statistical analysis
In all cell growth assays, the mean and standard deviation were calculated from 6 wells of sample and 12 wells of control. The statistical analysis was carried out with two-sample unpaired Student t-test performed using R (version 3.4.1; The R Foundation, Vienna, Austria) and 95% CI of the mean difference was plotted.
Results
Case LC-1
A 61-year-old man was diagnosed with lung adenocarcinoma and underwent surgical resection of the right middle lobe (pathological stage 1A). Four years later, the adenocarcinoma recurred in the right upper lobe and metastasized to multiple lymph nodes, bone and the adrenal glands. The patient received four cycles of cisplatin/pemetrexed/bevacizumab, followed by maintenance therapy with pemetrexed/bevacizumab. After five cycles of maintenance therapy, new metastases were found in the cervical lymph nodes. Following a cycle of pembrolizumab, the patient developed fever and hypoxemia owing to drug-induced pneumonia. The drug was discontinued and the patient was treated with oral steroids for 3 months. The BRAF V600E mutation was detected in the surgical specimen (LC-1 pre); thus, DT was administered as a third-line therapy. DT caused a temporary reduction in tumor size; however, the disease progressed to brain metastasis, meningitis and pleural effusion (LC-1 post) within 3 months. Whole-brain irradiation was performed, but the patient died 1 month after discontinuing DT.
Case LC-6
A 62-year-old man was diagnosed with lung adenocarcinoma by transbronchial lymph node biopsy with bronchoscopy. Because the patient had clinical stage 3B disease, four cycles of cisplatin/pemetrexed/bevacizumab were administered, followed by maintenance therapy with pemetrexed/bevacizumab. After seven cycles of maintenance therapy, the patient was switched to nivolumab as second-line therapy owing to right lung progression. After 19 cycles of nivolumab, the patient was switched to docetaxel/ramucirumab owing to right lung progression and lymph node metastasis. After 10 cycles of docetaxel/ramucirumab, the disease progressed with carcinomatous lymphangitis and bone metastasis. BRAF V600E was detected in a re-biopsy specimen of the mediastinal lymph nodes (LC-6 pre); thus, DT was started as a fourth-line therapy. After 11 months of DT, the disease progressed with pleural effusion (LC-6 post). The patient died 4 months after discontinuing DT.
Genomic analysis and protein expression
Next-generation sequencing was performed for LC-1 and LC-6 pre/post to evaluate acquired DT resistance (Figure 1, Table 1, Table S1). In LC-1, BRAF V600E was identified before and after DT treatment. The copy number variation (CNV) analysis showed that CDK4 on chromosome (chr) 12 was amplified after DT treatment. In LC-6, BRAF V600E was confirmed by SNV analysis before and after treatment, and missense S2264L and nonsense S2269* mutations in the AT-rich interaction domain 1A (ARID1A) were detected on chr 1 in the post-DT sample. In both LC-1 and LC-6, other copy number aberrations were detected; however, there was no focal amplification directly associated with resistance.
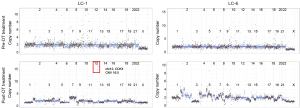
Table 1
| Cases | Chr | Gene name | Variant type | CNV change | Pre-DT treatment (copy number) | Post-DT treatment (copy number) |
|---|---|---|---|---|---|---|
| LC-1 | Chr12 |
|
CNV | Gain | 2 | 16.5 |
| Chr7 |
|
CNV | Gain | 2 | 3.5 | |
| Chr1 |
|
CNV | Loss | 2 | 1.0 | |
| LC-6 | Chr2 |
|
CNV | Gain | 2 | 4.0 |
| Chr5 |
|
CNV | Gain | 2 | 4.0 | |
| Chr1 |
|
CNV | Gain | 2 | 3.5 | |
| Chr3 |
|
CNV | Loss | 2 | 1 |
CNV, copy number variation; chr, chromosome; DT, dabrafenib and trametinib.
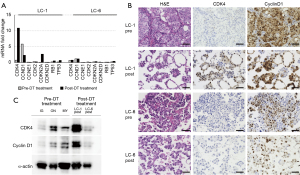
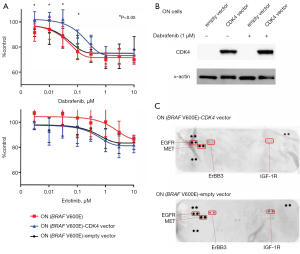
Next, we performed quantitative PCR and IHC analyses on LC-1 and LC-6 pre/post samples (Figure 2A,2B). In the LC-1 post sample, CDK4 mRNA was upregulated, as were the tumor suppressors CDKN2A (encoding p16), TP53, and Rb1. The CDK4 protein was upregulated after DT therapy in LC-1, whereas CCND1 mRNA (encoding cyclin D1) was downregulated. However, cyclin D1 expression was observed before and after treatment, and cell cycle signatures were not markedly altered in LC-6 (Figure 2A,2B). Western blotting results (Figure 2C) showed that CDK4 was expressed abundantly in LC-1, but only marginally in the other samples. Finally, we tested in vitro experiments to determine whether cells in the BRAF V600E cell line expressing the CDK4 protein are resistant to BRAF inhibitors. ON cells were transfected with a vector encoding CDK4 and treated with dabrafenib; at a concentration of 100 nM dabrafenib, ON cells transfected with the CDK4 gene were more resistant than controls (Figure 3A). On the other hand, cells treated with erlotinib, an EGFR inhibitor, did not show any suppression of proliferation. The expression of CDK4 protein was increased in ON cells transfected with a vector encoding CDK4 (Figure 3B). We further examined whether overexpression of CDK4 causes a bypass pathway for BRAF inhibitor resistance by increasing the phosphorylation of RTKs. The results showed that the phosphorylation of RTKs was not altered with or without forced expression of CDK4 protein (Figure 3C).
Discussion
Increased CDK4 DNA copy numbers followed by increased mRNA and protein levels may be associated with the early acquisition of DT resistance in BRAF V600E-mutated lung adenocarcinoma. Data from clinical trials show that the median duration of response to the DT combination DT is 9.8 months in previously treated patients with BRAF V600E-mutated NSCLC, and the clinical course of LC-6 was similar. In contrast, LC-1 showed a short response period of about 3 months and rapidly worsened, exhibiting carcinomatous pleurisy, brain metastasis, and meningitis at relapse. Thus, there may be an association with an unknown resistance mechanism involving CDK4.
Rudin et al. first reported resistance to dabrafenib in clinical specimens in BRAF V600E-mutated adenocarcinoma in 2013 (4), KRAS G12D mutations and CDKN2A nonsense mutations were the main causes of resistance. Previous case studies reporting acquired resistance to DT therapy in BRAF V600E-mutated adenocarcinoma have detected KRAS G12V and NRAS Q61K mutations. Facchinetti et al. (3) performed a genetic analysis of eight BRAF V600E-mutated lung cancers resistant to trametinib or dabrafenib and found mutations in MEK1, NRAS, KRAS and PTEN in four cases. Additionally, comparative genomic hybridization arrays were performed in the four cases in which no novel genetic mutations were identified by targeted next-generation sequencing, and deletions of CDKN2A/B were found in two of these cases. Thus, the mechanisms of resistance to BRAF or BRAF/MEK inhibition include reactivation of the RAS/MAPK pathway, supplementing signaling through other RAF family members, and activation of the PI3K/AKT pathway by PTEN deficiency. CDKN2 deletion or nonsense mutations are common in many cancers, although the significance of these mutations in the acquisition of DT resistance is unclear. Reactivation of the MAPK pathway and suppression of p16ink4 lead to positive regulation of the cell cycle; however, abnormalities in RAS and CDKN2 were not detected in our resistant cases.
Cyclin D-dependent kinases 4 and 6 are core proteins in the cell cycle machinery. In response to mitogenic signals, the cyclin D CDK4/CDK6/Cip/Kip complex is formed, resulting in the isolation of the Cip/Kip proteins from cyclin E-CDK2. Cyclin D- and E-dependent kinases phosphorylate Rb protein, releasing E2F, which is required for the transcription of genes necessary for S phase progression, including cyclin E itself, forming a positive feedback loop at the G1-S boundary. In malignant cells, alterations in the expression of CDK and its regulators, such as cyclin overexpression, regulate CDK activity and selectively promote proliferation (5). CDK4/6 inhibitors inhibit cell cycle progression and shows anti-tumor effects (6). Smalley et al. reported CDK4 mutations or CCND1 amplification in BRAF V600E-mutated melanomas and evaluated their roles in resistance to BRAF inhibition (11). They found that CCND1 amplification contributes to resistance to BRAF inhibition, particularly in the presence of CDK4 mutations or overexpression. However, because LC-1 harbored only CDK4 amplification, it is unclear whether the same resistance mechanism occurs in lung cancer.
The present in vitro study suggests that induction of CDK4 protein in BRAF V600E mutant cells results in dabrafenib resistance. The mechanism of resistance in lung cancer includes protein mutation due to the acquisition of gene mutation of therapeutic target and expression of bypass pathway mainly by RTKs. In these cells, the absence of secondary resistance mutations in BRAF has been confirmed by the results of comprehensive genetic analysis. Therefore, we examined the phosphorylation of RTKs with an antibody array and did not find any specific changes. A weakness of this in vitro study is that ON cells are partially resistant to BRAF inhibitors; the inhibition of cell proliferation by dabrafenib above 100 nM was about 70%, suggesting that this is a naturally acquired function during cell line establishment.
Taken together, these findings in LC-1 suggested that tumor cells originally harboring BRAF V600E mutations, and de novo or early acquired CDK4 amplification, selectively survived under simultaneous BRAF/MEK inhibition. We are currently investigating how CDK4 contributes to the mechanism of resistance. Unfortunately, it was not possible to establish cell lines with pre-DT-treated samples. In IHC, cell blocks were used for post-DT samples because tissue biopsy was clinically difficult in these cases. Immunostaining of cell blocks may overestimate the intensity of staining. However, changes in CDK4 staining of LC-1 were evident.
In summary, CDK4 may be involved in the early acquisition of resistance and a new target for overcoming such resistance.
Acknowledgments
The authors would like to thank Editage for English language editing.
Funding: This work was supported by JSPS KAKENHI Grant Number JP18K08132. The funding source had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-415
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tlcr-21-415
Peer Review File: Available at https://dx.doi.org/10.21037/tlcr-21-415
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-415). Dr. Yutaka Hatanaka reports lecture fees from AstraZeneca and Novartis and research funds from Sysmex and Thermo Fisher Scientific. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All study participants provided informed consent and this study was approved by the Institutional Review Board of Asahikawa Medical University (No: 18185/19081). Written consent was obtained from all patients whose samples were analyzed, including pleural effusions.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- Abravanel DL, Nishino M, Sholl LM, et al. An Acquired NRAS Q61K Mutation in BRAF V600E-Mutant Lung Adenocarcinoma Resistant to Dabrafenib Plus Trametinib. J Thorac Oncol 2018;13:e131-3. [Crossref] [PubMed]
- Facchinetti F, Lacroix L, Mezquita L, et al. Molecular mechanisms of resistance to BRAF and MEK inhibitors in BRAFV600E non-small cell lung cancer. Eur J Cancer 2020;132:211-23. [Crossref] [PubMed]
- Rudin CM, Hong K, Streit M. Molecular characterization of acquired resistance to the BRAF inhibitor dabrafenib in a patient with BRAF-mutant non-small-cell lung cancer. J Thorac Oncol 2013;8:e41-2. [Crossref] [PubMed]
- Cole AM, Myant K, Reed KR, et al. Cyclin D2-cyclin-dependent kinase 4/6 is required for efficient proliferation and tumorigenesis following Apc loss. Cancer Res 2010;70:8149-58. [Crossref] [PubMed]
- Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov 2016;6:740-53. [Crossref] [PubMed]
- Chen SH, Gong X, Zhang Y, et al. RAF inhibitor LY3009120 sensitizes RAS or BRAF mutant cancer to CDK4/6 inhibition by abemaciclib via superior inhibition of phospho-RB and suppression of cyclin D1. Oncogene 2018;37:821-32. [Crossref] [PubMed]
- Yadav V, Burke TF, Huber L, et al. The CDK4/6 inhibitor LY2835219 overcomes vemurafenib resistance resulting from MAPK reactivation and cyclin D1 upregulation. Mol Cancer Ther 2014;13:2253-63. [Crossref] [PubMed]
- Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res 2011;71:6051-60. [Crossref] [PubMed]
- Hirai N, Sasaki T, Okumura S, et al. Novel ALK-specific mRNA in situ hybridization assay for non-small-cell lung carcinoma. Transl Lung Cancer Res 2020;9:257-68. [Crossref] [PubMed]
- Smalley KS, Lioni M, Dalla Palma M, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther 2008;7:2876-83. [Crossref] [PubMed]


