Intrapulmonary lymph node retrieval: unclear benefit for aggressive pathologic dissection
Lymph node (LN) status is a highly significant component of staging non-small cell lung cancer (NSCLC). Staging of NSCLC provides prognostic data related to risk of recurrence as well as overall survival (1). Lymph node metastasis alters treatment decisions, including surgical resectability and appropriateness of adjuvant interventions. Current pathologic LN sampling techniques often evaluate a low number of LNs (2-4). Goals of current staging techniques include sampling at minimum three N1 (hilar) nodes examined in settings of resectable disease (5). Patients harboring N1 metastatic disease are a heterogeneous group with a calculated 5 year overall survival (OS) of 38% (1) with widely variable range from 27-67% (1,6,7).
In a recent article by Ramierz et al. (8), “Incomplete Intrapulmonary Lymph Node Retrieval After Routine Pathologic Examination of Resected Lung Cancer”, traditional pathologic LN staging was compared with a specialized technique for intrapulmonary LN sampling. The special pathologic examination (SPE) was used to detect additional LNs via re-dissection of 73 lobectomy (or greater) specimens following curative intent resection for NSCLC. Re-dissection consisted of 3-5 mm cuts of all discarded specimen following routine pathologic examination (RPE) with isolation of intrapulmonary LNs as well as identification of satellite metastatic nodules that did not meet LN criteria. Each SPE was compared to its own RPE, thus providing an internal control. All cases had appropriate pre-surgical staging. Of the 374 LNs examined with RPE 34 nodes harbored metastatic disease, a total of 22 of 73 patients. An additional 514 LNs were examined with the SPE technique with a median of 6 additional N1 nodes discovered, 4 from hilar/intralobar zone (range, 0-31) and 1 from peripheral zone (range, 0-15). Fifty six of the 514 nodes recovered with SPE were involved with metastatic disease, 20 of 73 patients. Of the 20 patients identified as having metastatic disease by SPE, 6 who were staged as N0 following RPE were found to have metastatic disease while 14 had previously been identified as having metastatic disease with RPE alone. A total of 8 of 73 patients (11%) were upstaged with alteration in management in 4 patients (5.4%). Two patients were upstaged from IIB to IIIA, one patient from stage IIA to IIIA (with malignant satellite nodules), and one from IIB to IIIA (with malignant satellite nodules). Median time to perform the SPE decreased from 44 minutes (range, 25-80 minutes) to 15 minutes (range, 10-30 minutes) in the last batch of 10 specimens examined. Lymph node retrieval rate remained stable despite this change in dissection duration.
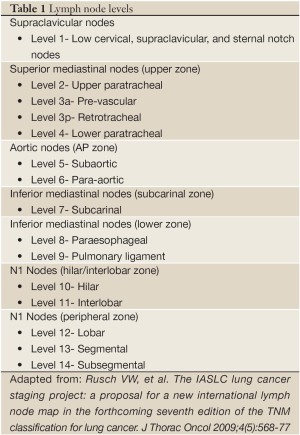
While a modest number of patients had upstaging from this technique (8 of 73), only half of these patients (4 of 73) had alteration to their therapy secondary to these findings. Furthermore, of the 4 patients with treatment alteration only 2 were secondary to N1 disease, the other 2 were found to have metastatic satellite lesions. Thus, it is difficult to deduce a survival advantage for the small number of patients who had an altered treatment based on the use of this intrapulmonary staging technique. Secondarily, drawing conclusions related to prognosis of the 8 patients with upstaged disease is difficult. The current staging system, and thus prognostic data, is based on our existing pathologic LN dissection techniques which often involves evaluation of few intrapulmonary nodes (9-11). Would we be treating these patients more aggressively without benefit? The question of ability to cross compare outcomes remains unanswered. However, interesting data published recently by Maeshima et al. (12) described prognostic implications for N1 nodal status. They stratified N1 nodal status based on hilar/interlobar zone (level 10-11 nodes) versus peripheral zone (level 12, 13 and 14 nodes) in 230 patients with pN1 disease in a Japanese population. Their study supported previous data that metastasis to high level nodes alone, segmental level 13 or subsegmental level 14, may have better prognosis (Table 1) (13-16). In their patient population those with hilar, level 11, or interlobar, level 12, nodal metastasis had a worse 5 year disease free survival (DFS) compared to level 13 or 14 involvement alone (P=0.021). If we examine the metastatic nodal level in the Ramirez et al. population (8) the number of peripheral zone LNs samples was indeed larger with SPE (0-3 with RPE vs. 0-15 with SPE). Yet the number of peripheral zone metastasis discovered remained essentially unchanged (RPE range, 0-1 vs. SPE range, 0-2). Suggesting that the SPE technique may not add significant data for prognosis related to DFS.
Full table
If we consider the study population based solely on the author’s initial hypothesis that current pathologic practice misses a significant number of intrapulmonary LNs, potentially harboring metastasis, does the quantity of intrapulmonary nodal metastasis discovered alter survival or prognosis? The authors clearly demonstrated an increased number of intrapulmonary positive nodes sampled through their technique as well as additional metastatic nodes discovered. SPE found a further 56 metastatic nodes in 20 of the 73 patients. Of these patients two thirds, 14 patients, were already identified as having metastatic disease via RPE increasing the total number of positive nodes identified for these patients. While, there is clear evidence that mediastinal LN sampling has impact on survival for N2 and N3 disease (9,17,18), controversy exists related to prognostic significance of number of N1 positive nodes. Jonnalagadda et al. (19) preformed a large retrospective population-based cohort evaluation through the use of the Surveillance, Epidemiology and End Results (SEER) database. They evaluated the impact of N1 disease and number of LN sampled. They concluded that a greater number of positive N1 nodes was an independent predictor of both lung cancer specific and overall survival (P<0.0001). Hazard ratio of lung cancer mortality with 2-3 positive N1 nodes was 1.6 (95% CI, 1.03-1.3), 4-8 positive N1 nodes was 1.53 (95% CI, 1.34-1.75), and greater than 8 positive N1 nodes was 2.25 (95% CI, 1.8-2.81). The risk of lung cancer specific death if greater than 8 N1 nodes were positive was equivalent to the findings of T3 disease. While this is not currently validated as a prognostic model, its results are compelling. Yet, recall that this data was for N1 disease which encompasses intrapulmonary nodes as well as ispilateral peribronchial and hilar nodal disease. It is not clear if these nodes were predominantly from an intrapulmonary source versus peribronchial or hilar nodes. Given the known sampling of intrapulmonary nodes in current surgical techniques being 3 or less (1,2) it is likely that a majority of the studied nodes were not intrapulmonary in origin. This makes it difficult to compare the data from this N1 nodal retrospective study with the Ramirez (8) study where the focus was intrapulmonary nodes alone. Moreover, several prior studies have conflicting data regarding degree of N1 nodal disease and its prognostic significance (20-22), although some had a heterogeneous population or small sample size.
Lastly, regarding performance of the SPE, the dissection duration time was the rate limiting step in evaluation of the specimen. The time to complete SPE, decreased as pointed out by the authors, with subsequent dissections. Yet, they described the dissections as being preformed as ‘batches’ of 10. Suggesting their pathology department preformed SPE in a repetitive manner which could lead to continued accuracy despite drop in dissection time, as the operator completed further evaluations. One must question whether this drop in time with consistent accuracy would hold true for the community hospital where these dissections would not be done on a daily basis. Furthermore, the cost in time for training as well as personnel and resources must be taken into consideration.
In the setting of new surgical, pathologic or diagnostic techniques we must ensure that there is benefit to the patient for employing these techniques. If no benefit can be clearly stated we must question if the cost in not only dollars but time and resources is appropriate for universal implementation. Certainly the author’s hypothesis held true. The specialized pathologic sampling technique did, indeed, discover additional metastatic disease. Yet, a significant piece of the diagnostic paradigm is missed. Their conclusion that adopting this improved LN dissection technique should be placed into routine practice may be putting the cart before the horse. The improved accuracy of staging that this technique affords, does not necessarily translate into functional data for the average clinician. Would the SPE staged patients be comparable with regards to outcomes and prognosis with their traditionally staged counterparts? As illustrated above, it is unclear if the upstaging of patients based on this technique will provide a survival advantage or prognostic data. A growing body of research evaluating the ways in which we stage, treat, and counsel our N1 patients continues to grow. The framework for continued evaluation of these topics is strengthened by the authors. Nonetheless without data regarding the impact related to patient survival or prognosis implementation of this new technique, outside of the research setting, is too far-reaching for universal adoption.
Acknowledgements
Disclosure: The authors have no conflict of interest to disclose.
References
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Gephardt GN, Baker PB. Lung carcinoma surgical pathology report adequacy: a College of American Pathologists Q-Probes study of over 8300 cases from 464 institutions. Arch Pathol Lab Med 1996;120:922-7.
- Farooq A, Osarogiagbon UR, Allen WJ, et al. Accuracy and comprehensiveness of pathology reportage after lung cancer resection. J Clin Oncol 2009;27:abstr 6523.
- Sobin L, Gospodarawics M, Wittekin C. eds. International Union Against Cancer: TNM Classification of Malignant Tumors. 7th ed. New York: John Wiley & Sons, Inc., 2009.
- Allen JW, Farooq A, O’Brien TF, et al. Quality of surgical resection for nonsmall cell lung cancer in a US metropolitan area. Cancer 2011;117:134-42.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14.
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7.
- Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol 2012;30:2823-8.
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70.
- Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest 2011;139:1124-9.
- Osarogiagbon RU, Allen JW, Farooq A, et al. Outcome of surgical resection for pathologic N0 and Nx non-small cell lung cancer. J Thorac Oncol 2010;5:191-6.
- Maeshima AM, Tsuta K, Asamura H, et al. Prognostic implication of metastasis limited to segmental (level 13) and/or subsegmental (level 14) lymph nodes in patients with surgically resected nonsmall cell lung carcinoma and pathologic N1 lymph node status. Cancer 2012;118:4512-8.
- van Velzen E, Snijder RJ, Brutel de la Rivière A, et al. Lymph node type as a prognostic factor for survival in T2 N1 M0 non-small cell lung carcinoma. Ann Thorac Surg 1997;63:1436-40.
- Riquet M, Manac’h D, Le Pimpec-Barthes F, et al. Prognostic significance of surgical-pathologic N1 disease in non-small cell carcinoma of the lung. Ann Thorac Surg 1999;67:1572-6.
- Caldarella A, Crocetti E, Comin CE, et al. Prognostic variability among nonsmall cell lung cancer patients with pathologic N1 lymph node involvement. Epidemiological figures with strong clinical implications. Cancer 2006;107:793-8.
- Demir A, Turna A, Kocaturk C, et al. Prognostic significance of surgical-pathologic N1 lymph node involvement in non-small cell lung cancer. Ann Thorac Surg 2009;87:1014-22.
- Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg 2008;85:211-5.
- Su X, Wang X, Long H, et al. Mediastinal lymph node dissection affects survival in patients with stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2008;56:226-30.
- Jonnalagadda S, Smith C, Mhango G, et al. The number of lymph node metastases as a prognostic factor in patients with N1 non-small cell lung cancer. Chest 2011;140:433-40.
- Martini N, Flehinger BJ, Nagasaki F, et al. Prognostic significance of N1 disease in carcinoma of the lung. J Thorac Cardiovasc Surg 1983;86:646-53.
- Marra A, Hillejan L, Zaboura G, et al. Pathologic N1 non-small cell lung cancer: correlation between pattern of lymphatic spread and prognosis. J Thorac Cardiovasc Surg 2003;125:543-53.
- Nakagawa T, Okumura N, Kokado Y, et al. Retrospective study of patients with pathologic N1-stage II non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2007;6:474-8.


