
Predictive value of mRNA expression and dynamic changes from immune related biomarkers in liquid biopsies before and after start of pembrolizumab in stage IV non-small cell lung cancer (NSCLC)
Introduction
Immune-checkpoint inhibitors (ICI) directed against programmed death 1 (PD-1), programmed death ligand 1 (PD-L1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have expanded the treatment options for advanced NSCLC. In patients without drugable driver mutations ICI were first approved for palliative 2nd and 3rd line treatment of metastatic NSCLC (1-5). Up to 16% of NSCLC patients receiving an ICI as palliative 2nd or 3rd line treatment survive for more than five years (6). Nowadays, ICI either alone or as combination therapy with chemotherapy are established in the 1st line setting. Especially, the PD-1 ICI pembrolizumab is approved in Europe as a 1st line monotherapy for patients with an immunohistochemically based tumor proportion score (TPS) for PD-L1 ≥50% after data from the registrational phase III trial KEYNOTE-024 showed superior results in comparison to a chemotherapy only control arm in terms of its primary endpoints PFS and OS (7). Recently, 5-year survival data were reported with 31.9% of patients living after 1st line treatment with this ICI in contrast to 16.3% with a chemotherapy only first-line treatment (HR 0.62) (8). However, in about one third of patients there is no long lasting benefit due to early progression, hyperprogression or serious adverse events (9). Therefore, it would be of utmost importance to have reliable predictive factors showing non-effectiveness of ICIs before the first radiological measurement of response is possible.
Beside PD-L1 tumor proportional score (TPS) analyzed immunohistochemically from the tissue biopsy before treatment there is no officially accepted clinical factor or biomarker to date (10). Various biomarkers including high tumor mutational burden (TMB) have been tested and reported to be associated with a better response rate and an increased survival (11). However, there is a lack of standardization between the testing platforms used and a lack of a fixed TMB threshold so far (12). Therefore, blood-based biomarkers, which can be obtained noninvasively and can easily be repeated came into focus (10). Using peripheral blood laboratory values, e.g., C-reactive protein (CRP) or the neutrophile-lymphocyte ratio (NLR) might be of predictive value during monotherapy with ICI (13,14). It was shown that both a decrease in CRP values and a decrease in the NLR ratio were associated with a good outcome in terms of PFS and even OS (14,15). However, these markers are rather unspecific with regard to response of specific immune cell types being affected by the therapy as they cannot distinguish, e.g., regulatory T-cell expansion from natural killer cell expansion upon ICI treatment, which might indicate inverse reactions and outcome. In line with these assumptions, dynamic changes of molecular markers in a so called liquid biopsy (LB), e.g., loss of ctDNA during ICI therapy or increase in CD8+PD-1+ cells in the peripheral blood might be of essential relevance as they reflect the very early response (16-20). However, those studies are hardly comparable due to small case numbers, patients in different lines of treatment with different PD-1 and PD-L1 directed antibodies and different time intervals used for the observation of the dynamic change. Moreover, FACS based methodologies are too complex for clinical routine and are sensitive to pre-analytical sample preservation and of limited robustness in view of reagent variations.
Therefore, we aimed to analyze immune-related markers in a homogenous cohort of NSCLC stage IV patients with high TPS score of at least 50% in their tumors being treated by the PD-1 antibody pembrolizumab in a 1st line setting. Whole blood samples were prospectively taken before start of ICI and after three weeks of treatment. In this analysis we report on pre-treatment mRNA expressions and dynamic changes of CD3, CD8, PD-1, PD-L1 and CTLA-4 after the first application of pembrolizumab using a commercially available kit. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-587).
Methods
Design of the study
This retrospective analysis studies predictive markers for efficacy under a palliative 1st line treatment with the ICI pembrolizumab in patients with metastatic NSCLC. Peripheral blood samples were taken prospectively before and during treatment with pembrolizumab. Patients had to have a histologically proven NSCLC UICC stage IV according to the 8th edition of the UICC TNM classification. Patients with a history of adjuvant chemotherapy before 1st line palliative therapy could be included if there was an interval of more than 12 months between the adjuvant treatment and the first systemic treatment for stage IV disease. Patients with a history of palliative radiation therapy could also be enrolled into the study. NSCLC with a non-squamous histology were tested for EGFR mutations and ALK translocations and were excluded in case of a positive result. Patients had to have a PD-L1 TPS score from the tumor biopsy of ≥50% to qualify for 1st line pembrolizumab according to the approval status of pembrolizumab in Germany.
Treatment was performed at a German tertiary high-volume lung cancer center (Nuremberg) with specialization in thoracic oncology and strong experience in chemo- and immune-oncology therapy. Patients were treated with pembrolizumab between February 2017 and October 2020, which allowed for a follow-up of at least six months prior to the data cut-off of April 30th, 2021. Data were recorded in a standardized manner with the following data being collected for each patient: age, sex, tumor stage, histology, performance status according to Eastern Cooperative Oncology Group performance status (ECOG-PS), PD-L1 tumor proportional score (TPS) determined by immunohistochemistry (marker ZR3), mutational status (for non-squamous tumors) determined by next-generation sequencing (NGS), date of NSCLC diagnosis, survival status at time point of documentation (alive or deceased) and date of last contact/death. For ICI treatment the following details of therapy were documented: date of start, number of treatment cycles, best response, date of progression and reason for treatment stop.
This study was approved by the Ethics committee of the Friedrich-Alexander-University Erlangen-Nuremberg, Germany (numbers: 56_16B and 62_17B). Written informed consent for blood taking, collecting and analyzing for research purpose was obtained from every patient. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
PD-L1 immunohistochemistry
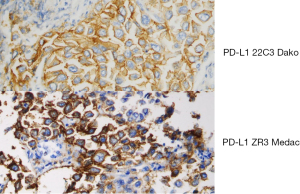
The tissue slides were incubated with antibodies against PD-L1 (clone ZR3; Medac, Wedel, Germany; 1:100 in dilution) using the Dako Agilent Link 48 auto-stainer according to the manufacturer’s standard recommendations. PD-L1 expression on tumor cells was counted by scoring the proportion of any membranous positive tumor cells over the total number of tumor cells (tumor proportion score, TPS), and grouped into four categories (<1% negative, 1–49% weak positive, ≥50–74% strong positive and ≥75 very strong positive). In addition, the exact percentage of the TPS was noted (21,22). The antibody ZR3 had been validated against 22C3 at our department and concordance with 22C3 was very high. An example of the immunohistochemical staining with both antibodies is given in Figure 1. In addition, after passing two German external quality assessments for PD-L1 staining the pathological department received approval with ZR3. In contrast to FDA companion diagnostic with 22C3 for use of pembrolizumab is not prescribed by the EMA in Europe.
RNA isolation and quantitative reverse transcription-polymerase chain reaction (QRT-PCR assessment)
RNA was extracted from whole blood samples according to general instruction for use of the commercially available bead-based extraction method (Xtrakt kit; Stratifyer Molecular Pathology GmbH, Cologne, Germany). In brief, 100 µL blood were put into a 1.5 mL microcentrifuge tube and 100 µL red cell lysis buffer was added to incubate for 15 minutes at 95 °C with shaking at 100 rpm in a thermomixer. Thereafter, the lysate was treated with proteinase K for 15 min at 65 °C. Subsequently binding buffer and magnetic beads were added and incubated for 15 min at room temperature with shaking at 1,200 rpm. Supernatant was discarded and the beads were washed by three cycles of adding wash buffer, aspiration, magnetization and discarding the supernatant. Finally, nucleic acids were diluted by adding 100 µL elution buffer and incubation at 95 °C for 15 min while shaking at 1,000 rpm. Thereafter, the beads were magnetized and the supernatant was DNAse I digested to receive DNAse free total RNA from the specimen. RNA eluates were then stored at −80 °C until use. The mRNA levels of CD3Z, CD8A, PD-1, PD-L1. CTLA-4 and the reference genes Calmodulin2 (CALM2) and Beta-2 microglobulin (B2M) were determined by a one-step RT-qPCR using the SuperScript III RT-qPCR system (Invitrogen, Waltham, MA, USA) and gene specific primer-probe combinations (Assay number MP317, MP769, MP675, MP676, MP501 and MP810, respectively; STRATIFYER Molecular Pathology GmbH, Cologne, Germany).
Each patient sample or control was analyzed in duplicate in a Light Cycler LC480 Instrument II (Roche Diagnostics, Rotkreuz, Switzerland) according to the manufacturers’ instructions with 30 min at 50 °C, 2 min at 95 °C followed by 40 cycles of 15 s at 95 °C and 30 s at 60 °C. according to MammaTyper® instructions for use 140603-90020-EU Rev 2.0. Gene expression was quantified with a modification of the method by Schmittgen and Livak by calculating 40−∆Ct, whereas ∆Ct was calculated as the difference in Ct between the test gene and the mean of the reference genes (23). Gene expression levels were calculated as described before. In short, cycle quantification threshold (Cq) values of marker genes (MG) for each sample (S) were estimated as the median of the triplicate measurements. To correct for inter-run variations Cq values were normalized against the mean expression of the REF genes and set off against a calibrator (PC) (ΔΔCq method). By subtracting ΔΔCq from the total number of cycles [40] it was ensured that normalized gene expression is proportional to the corresponding mRNA expression levels. This method facilitates interpretation of data and clinicopathological correlations. The various calculation steps are summarized in the following formula: 40−ΔΔCq(MG)S = 40 − {[Cq(MG)S − meanCq(REF)S] − [Cq(MG)pc − meanCq(REF)pc]}. Finally, the individual biomarker dynamics upon ICI treatment was determined by subtracting post-treatment expression values from pre-treatment expression values (Δ expression).
Objective cut-off points for high and low expression were determined by the median expression of each marker.
Statistical analysis
The primary endpoint was efficacy of pembrolizumab given 1st line in terms of progression-free survival (PFS). Secondary endpoints were OS from start of 1st line therapy and best response. Markers were evaluated for their predictive value for these endpoints.
Tumor responses were assessed by thoracic computed tomography (CT) and abdominal ultrasound or other clinically appropriate abdominal imaging technique at least every 3 months or upon clinical deterioration. Tumor responses were evaluated according to the RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1 (24). Complete response (CR) was defined as disappearance of all target and non-target lesions. Partial response (PR) was defined as ≥30% reduction in size in target lesions or disappearance of ≥1 non-target lesions. Stable disease (SD) was defined as <30% decrease or <20% increase in size of target lesions or the persistence of ≥1 non-target lesions. Progressive disease (PD) was defined as ≥20% increase in size or the appearance of new non-target lesions and/or progression of existing non-target lesions. The ORR was defined as the best response recorded from the start of treatment until disease progression or recurrence, confirmed by repeat assessments performed no less than four weeks after the first timepoint when criteria for response had been reached. Disease control rate was defined as percentage of patients with CR plus PR plus SD. Overall survival was recorded from the first day of 1st line palliative treatment with pembrolizumab to the date of death or last follow-up. PFS was defined as the interval from the first day of 1st line treatment to the first sign of disease progression or death whichever occurred first. Subsequent therapies were documented.
Descriptive data are presented as median and 95% confidence interval, categorical variables are presented using numbers and frequencies. PFS and OS are presented as median and 95% confidence interval (CI), and times to events were determined using the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazards models were used to evaluate the influence of different patient factors. Statistical results were calculated using SPSS® (IBM; version 23). A P value <0.05 was considered statistically significant.
Results
Patient population
After excluding 7 patients treated with pembrolizumab 1st line at our center (4 patients with insufficient blood samples for and after start of ICI treatment and 3 patients with insufficient follow-up data), 45 patients met the inclusion criteria. The baseline demographic data and tumor characteristics are listed in Table 1. The median age was 68 years (range, 49–82 years), and 57.8% of the patients were male. More than half of the patients had an Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0 (58.1%). PD-L1 immunohistochemistry data were available for all patients. All patients had a TPS score of ≥50%. Of those 48.9% and 51.1% presented with a TPS score of ≥50% and <75% or ≥75%, respectively.
Table 1
| Parameters | N (%) |
|---|---|
| Gender | |
| Male | 26 (57.8) |
| Female | 19 (42.2) |
| Age | |
| <65 years | 11 (24.4) |
| ≥65 years | 34 (75.6) |
| Histology | |
| Adeno-ca | 28 (62.2) |
| Squamous cell ca | 13 (28.9) |
| NOS | 4 (8.9) |
| Stage at start of ICI | |
| Stage IVA | 11 (24.4) |
| Stage IVB | 34 (75.6) |
| ECOG-PS | |
| 0 | 25 (58.1) |
| 1 | 18 (41.9) |
| PD-L1 TPS | |
| <75% | 22 (48.9) |
| ≥75% | 23 (51.1) |
NOS, not other specified; ICI, immune-checkpoint inhibitor; ECOG-PS, Eastern Cooperative Oncology Group performance status; TPS, tumor proportion score.
Outcome of the whole cohort
After a median follow-up of 27.4 months an ORR to 1st line therapy was observed in 66.7% of patients with a median duration of response (DOR) of 34.1 months (95% CI: 20.6–47.6). Disease control rate was 68.9% with 22% showing a progressive disease (PD) (Table S1). After a median number of 11 cycles of pembrolizumab (range, 2–38) the median PFS was 13.2 months (95% CI: 3.4–23.1) with 6-, 12- and 24-month PFS rates of 66.7%, 50.4% and 44.4%, respectively. Six patients (13.3%) were still on treatment. From the start of ICI the median OS was 29 months (95% CI: not reached) with 12- and 24month survival rates of 65.7% and 59.4%, respectively (Figure 2). In addition, clinical subgroups were tested for predictive value in terms of PFS or OS. Beside histologic type of NSCLC (adenocarcinoma vs. squamous cell carcinoma or NOS) which determines a significant difference in PFS (P=0.033) and a trend for OS (P=0.061) none of the other parameters were of significant predictive value (Table 2). While type of histology was a significant parameter for PFS in the univariate cox-regression analysis it did not remain significant in multivariate analysis (data not shown).
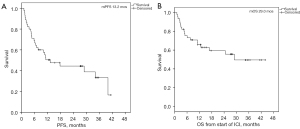
Table 2
| Parameters | PFS | OS | |||
|---|---|---|---|---|---|
| Median, months | P value | Median, months | P value | ||
| Gender | 0.076 | 0.871 | |||
| Male | NR | NR | |||
| Female | 6.4 | 29.0 | |||
| Age | 0.383 | 0.111 | |||
| <65 years | NR | NR | |||
| ≥65 years | 11.2 | 24.6 | |||
| Histology | 0.033 | 0.061 | |||
| Adeno-ca | 34.1 | NR | |||
| Squamous cell ca | 10.1 | 12.7 | |||
| NOS | 4.4 | 6.1 | |||
| Stage at start of ICI | 0.447 | 0.769 | |||
| Stage IVA | NR | NR | |||
| Stage IVB | 11.1 | 29.0 | |||
| ECOG-PS | 0.325 | 0.180 | |||
| 0 | 34.1 | NR | |||
| 1 | 11.1 | 12.7 | |||
| PD-L1 TPS | 0.401 | 0.226 | |||
| <75% | 6.9 | 11.2 | |||
| ≥75% | 17.8 | 29.0 | |||
PFS, progression-free survival; OS, overall survival; NR, not reached; NOS, not other specified; ICI, immune-checkpoint inhibitor; ECOG-PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death-ligand 1; TPS, tumor proportion score.
Blood-based gene expression before start of ICI treatment and survival
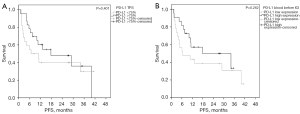
To identify predictive peripheral blood biomarkers for pembrolizumab treatment, we examined PD-1, PD-L1, CTLA-4, CD3 and CD8 before treatment. Relative gene expressions divided by their median in low and high expression revealed no significant difference for ORR, PFS or OS in log rank test (Table 3 and Table S2). Though not statistically significant, it was interesting to see, that PD-L1 TPS scores (<75% vs. ≥75%) from the tumor tissue and PD-L1 expression (low versus high expression by median) from the LB revealed similar mPFS rates and Kaplan-Meier curves; the corresponding mPFS rates were 6.9 vs. 17.8 months (TPS score P=0.401) and 6.4 and 17.8 months (gene expression P=0.262) for low and high expressers, respectively (Figure 3). Comparing mRNA markers with general blood/serum marker (leucocytes, neutrophils, CRP or LDH) indicated that the quantitative assessment by molecular analysis of CD3, CD8, PD-1, PD-L1 and CTLA4 identifies a subset of immune cells, which does not relate to total amount of less characterized immune cell types. While no correlation of the mRNA levels of immune markers could be found with LDH, there were some modest positive Spearman correlations (r=0.26 to r=0.38) when comparing mRNA markers with lymphocyte counts, which did not reach statistical significance (P=0.07 to P=0.21) (data not shown).
Table 3
| Parameters | PFS | OS | |||
|---|---|---|---|---|---|
| Median, months | P value | Median, months | P value | ||
| CD3 | |||||
| Low | 11.2 | NR | |||
| High | 13.2 | 0.981 | 29.9 | 0.960 | |
| CD8 | |||||
| Low | 6.4 | 29.0 | |||
| High | 13.2 | 0.730 | NR | 0.313 | |
| CTLA-4 | |||||
| Low | 11.2 | NR | |||
| High | 13.2 | 0.638 | 29.0 | 0.553 | |
| PD-1 | |||||
| Low | 11.1 | 11.2 | |||
| High | 17.8 | 0.919 | NR | 0.084 | |
| PD-L1 | |||||
| Low | 6.4 | 29.0 | |||
| High | 17.8 | 0.262 | NR | 0.198 | |
PFS, progression-free survival; OS, overall survival; NR, not reached; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed cell death protein 1; PD-L1, programmed cell death-ligand 1.
Difference of blood-based gene expression before and after start of ICI treatment and survival
Next, we performed measurements of the five above mentioned biomarkers after start of ICI treatments. Pre- and post ICI mRNA expressions were comparable due to 40−ΔCt values, were all in the same range and therefore, highly comparable. In addition, to evaluate a dynamic change, we categorized patients for every marker into decreased expression (Δ ≤0) and increased expression (Δ >0) post treatment groups.
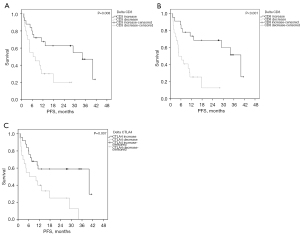
ORR and PFS were significantly improved by patients with increased whole blood expression of CD3 and CD8 within 3 weeks after start of pembrolizumab therapy (Table 4 and Table S3). Interindividual CD3 and CD8 changes from baseline in association to the median PFS are shown in Figure S1. Especially, CD 8 revealed to be a good predictive discriminator with mPFS rates of 40.0 months (increase) versus 4.7 months (decrease) (P<0.001) (Figure 4). The same strong predictive value was observed for OS where the intra-individual change CD8 exhibited mOS rates of NR vs. 11.1 months (P<0.001) Similarly, intraindividual change in CD 3 expression was of predictive value in terms of OS with mOS rates of NR vs. 12.7 months for increase versus decrease of this marker, respectively (Figure 5). In contrast, changes of PD-1 and PD-L1 were not predictive, neither for PFS nor OS (Table 4).
Table 4
| Parameters | mPFS | mOS | |||
|---|---|---|---|---|---|
| Median, months | P value | Median, months | P value | ||
| Δ CD3 | |||||
| Increase | 34.1 | NR | |||
| Decrease | 6.4 | 0.008 | 12.7 | 0.022 | |
| Δ CD8 | |||||
| Increase | 40.0 | NR | |||
| Decrease | 4.7 | <0.001 | 11.1 | <0.001 | |
| Δ CTLA-4 | |||||
| Increase | 40.0 | NR | |||
| Decrease | 6.4 | 0.007 | 16.8 | 0.087 | |
| Δ PD-1 | |||||
| Increase | 29.0 | NR | |||
| Decrease | 7.6 | 0.083 | 16.8 | 0.319 | |
| Δ PD-L1 | |||||
| Increase | 29.0 | n.r. | |||
| Decrease | 11.1 | 0.395 | 24.6 | 0.365 | |
Δ, change in expression before and after start of immune-checkpoint inhibitor. PFS, progression-free survival; OS, overall survival; NR, not reached; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed cell death protein 1; PD-L1, programmed cell death-ligand 1.
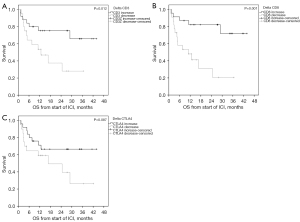
Finally, we performed univariate and multivariate analyses to test Δ expressions for independent predictive value. Positive delta CD8 values became the only independent predictive marker for as well PFS as OS with P values of 0.011 and 0.006, respectively (Tables 5,6).
Table 5
| Parameters | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Δ CD3 (increase vs. decrease) | 2.937 | 1.273–6.777 | 0.012 | 0.393 | 0.084–1.849 | 0.237 | |
| Δ CD8 (increase vs. decrease) | 4.438 | 1.789–10.954 | <0.001 | 7.748 | 1.614–37.277 | 0.011 | |
| Δ PD-L1 (increase vs. decrease) | 2.876 | 1.292–6.398 | 0.010 | 2.327 | 0.942–5.746 | 0.067 | |
| Δ CTLA-4 (increase vs. decrease) | 1.990 | 0.902–4.392 | 0.088 | ||||
| Δ CD3 (increase vs. decrease) | 2.876 | 1.292–6.398 | 0.010 | ||||
Δ, change in expression before and after start of immune-checkpoint inhibitor. PFS, progression-free survival; PD-1, programmed cell death protein 1; PD-L1, programmed cell death-ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4.
Table 6
| Parameters | OS | ||||||
|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Δ CD3 (increase vs. decrease) | 2.920 | 1.124–7.585 | 0.028 | 0.467 | 0.109–1.996 | 0.302 | |
| Δ CD8 (increase vs. decrease) | 5.010 | 1.740–14.420 | 0.003 | 9.747 | 1.900–50.013 | 0.006 | |
| Δ CTLA-4 (increase vs. decrease) | 2.181 | 0.874–5.442 | 0.095 | ||||
| Δ PD-1 (increase vs. decrease) | 1.582 | 0.636–3.932 | 0.323 | ||||
| Δ PD-L1 (increase vs. decrease) | 1.514 | 0.613–3.740 | 0.369 | ||||
Δ, change in expression before and after start of immune-checkpoint inhibitor. OS, overall survival; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed cell death protein 1; PD-L1, programmed cell death-ligand 1.
Discussion
In this study we could demonstrate that intraindividual dynamic changes in mRNA expressions of CD3 and CD8 occur after the first application of pembrolizumab in therapy naïve NSCLC patients and are predictive for better PFS and OS. CD8 expressing cells being positive for PD-1 (CD8+PD-1+) are a subpopulation of T lymphocytes which are exhausted in tumors where the PD-1 pathway leads to a tumor immune escape (25). The mode of action of immune-checkpoint inhibitors is the blockade of the PD-1 pathway leading to a reinvigoration of T cells with proliferation of CD8+PD-1+ T cells (16). The proliferation of those T cells recovers their functions and leads to tumor infiltration (26). In melanoma it could be shown that these CD8+PD-1+cells were functional cytotoxic T cells targeting cancer and their increase after ICI treatment had been associated with tumor response (27).
In line with our findings are analyses in the peripheral blood of NSCLC patients responding to PD-1 directed ICI therapy where an association with early proliferation of CD8+PD-1+ T cells could be detected. A study by Kamphorst et al. described that those dynamic changes occurred in Ki-67+CD8+PD-1+ T cells from the peripheral blood of 29 NSCLC patients before and after start of ICI therapy (17). An increase after the first or second course of treatment at a 2–6 weeks’ time interval was associated with better clinical response. Ki-67+CD8+PD-1+ T cells have also been measured in another cohort of 79 NSCLC patients before and during ICI treatment (18). An increase in those cells in the peripheral blood at the first week of PD-1 directed therapy was also associated with radiological tumor response. In addition, those patients had a favorable outcome in terms of PFS and OS. Very recently, Yamauchi et al. described CX3CR1 as another marker on CD8+ T cells being involved in response to a PD-1 directed therapy in NSCLC patients (20). After proof of principle in mice the peripheral blood of 36 human patients was studied for dynamic changes of this T cell marker. In line with the tumor-bearing mice results an increase in CXCR1+CD8+ T cells after start of ICI was associated with response and a clinically relevant better PFS. In summary, the increase in CD8 mRNA detected in the peripheral blood of NSCLC patients responding to pembrolizumab in our cohort supports findings of basic research studies and might be an easy to obtain surrogate marker of proliferating CD8+PD-1+ T cells.
Likewise, a dynamic increase in expression of CD3 was associated with a favourable outcome in terms of PFS. The marker measured by us was CD3Z, a sequence being part of the T-cell receptor (TCR) (28). Activation of the host immune response against tumor cells includes the recognition of neoantigens by proliferating TCRs (16,29). Thus, changes in the clonality or repertoire of the TCR might predict response to ICI (30). In a study by Han et al., the TCR repertoire diversity of CD8+PD-1+ T cells in the peripheral blood was measured before and 4–6 weeks after ICI therapy in 40 NSCLC patients (16). Interestingly, they could show that both an increased clonality as well as diversity of the TCR were associated with better response and longer survival. These data underline that dynamic changes in a subset of CD8 and CD3 cells occur very early after start of an ICI directed therapy and can be detected via circulating mRNA in the blood.
To our knowledge there are only few reports published so for on mRNA expressions of immune-related genes from whole blood samples obtained from peripheral blood before and during start of pembrolizumab treatment in NSCLC. Till now, attention of basic research engaged in liquid biopsy is mainly directed on CD8+ T cells which was reported above and needs laborious cell sorting techniques. In contrast, mRNA seems to be robust and gene expressions are quite easily to measure (31). In a study by Yang et al. published very recently, mRNA expression in blood plasma was analyzed in 33 patients before and two months after initiation of ICI therapy in NSCLC (32). They could show that increased expression of PD-L1 mRNA (≥2.04 fold-change) was correlated as well with clinical response and better outcomes in terms of OS and PFS. Their data are in line with our results were dynamic changes of PD-L1 and PD-1 occurred during ICI treatment. While our data point the same direction, with higher expressions indicating a good prognosis, our data were not statistically significant in this part of our analyses. However, in contrast to the study by Yang et al. we measured dynamic changes after three weeks which might be the cause of underscoring the effect of increase of PD-L1 expression over time. In addition, patients in our cohort had to have a tissue PD-L1 TPS of ≥50% to be eligible for pembrolizumab treatment. As PD-L1 ≥50% measured by IHC is described as a marker of good response and outcome, all our patients were already in this group and dynamic changes might not be of such relevance as in the cohort of Yang et al. where the whole range of the PD-L1 TPS was included. Interestingly, patients in our study presenting with a baseline PD-L1 mRNA expression above the median had increased survival data in terms of PFS in contrast to patients with lower expressions with median PFS of 17.8 and 6.4 months, respectively. These data were highly comparable to the immunohistochemical tissue data of the same patients with a TPS score dichotomized into ≥75% vs. <75% with median PFS rates of 17.8 and 6.9 months, respectively. This indirect comparison may be a hint of the robustness and evaluability of mRNA measured in whole blood samples.
Our study suggests that changes in CD8 mRNA expression immediately after the first course of an ICI may easily identify patients with a high chance of good clinical response to this ICI monotherapy. In the KEYNOTE-24 trial a 6-months PFS rate of 62.1% was described for the pembrolizumab arm (7). This result is very comparable with the 66.7% of patients in our cohort remaining on treatment after 6 months. In reverse, these data indicate that about one third of patients will have clinical progression within the first 6 months of treatment. Those non-responsive patients might be treated by combining ICI with platinum-based chemotherapy. Based on our data the switch from ICI monotherapy to ICI-chemotherapy could be initiated as early as the second course of ICI. Data from the KEYNOTE 189 and KEYNOTE 407 showed that a combination therapy is superior to a chemotherapy only regimen in terms of PFS and OS in squamous and non-squamous carcinoma, respectively (33,34). After reaching both endpoints pembrolizumab plus platinum-based chemotherapy regimen are approved for squamous and non-squamous NSCLC patients regardless of the PD-L1 TPS status. In addition, a combination therapy including platinum-based combination therapy in combination with a PD-1 (nivolumab) and a CTLA-4 directed antibody (ipilimumab) were also approved in this setting as the registrational phase III trial 9LA had been positive for its primary endpoint OS (35). However, whether a rapid change after one cycle of therapy from pembrolizumab monotherapy to ICI plus chemotherapy is associated with clinical benefit has to be shown as there is not even a head-to-head comparison between ICI based mono- and ICI plus chemotherapy combination-regimen to date.
Limitations of this study include the retrospective design, the lack of an independent validation cohort, and the small sample size. Another important limitation is the fact that the mRNA was measured from whole blood and therefore, it is not clear from which exact subtypes of immune cells it may have been released. Though mRNA seems to be more robust in blood as originally adopted, and ct-values could be consistently repeated, independent cohorts are necessary to confirm these results. Furthermore, we do not know whether there are dynamic changes also at the time of progression of patients initially responded to pembrolizumab. This will be part of a further research project. At least, patients included in this study had to have two time points in a 3-weeks interval where blood was taken for mRNA analyses. All patients with just one liquid biopsy probe taken before ICI treatment were excluded. Therefore, there might be a lack in terms of missing poor performing individuals after one cycle of pembrolizumab. However, as our 6-months PFS data are comparable with the KEYNOTE-24 study a relevant bias might be excluded.
In conclusion, in this study we demonstrate that mRNA expression of immune-related genes can be measured from whole blood samples during ICI and dynamic changes with increase of CD8, CD3 and CTLA-4 mRNA expression three weeks after start of pembrolizumab were associated with favorable outcome in terms of PFS and OS. Especially, a dynamic chance in CD8 expression with increase after the first application of ICI was an independently predictive for better PFS and OS. These results, if confirmed in a larger cohort, demonstrate a potential role for serial mRNA analyses of these markers as a new early indicator of clinical efficacy of ICI therapy. In particular, the dynamic changes in immune-related mRNA expressions could allow earlier prediction of clinical benefit compared with radiological imaging. Patients who do not respond to pembrolizumab monotherapy might thus be identified earlier, so that their therapy can then be modified earlier than before. Early escalation of such non-responsive patients for instance to chemo-ICI combination therapy might improve their outcomes significantly.
Acknowledgments
We thank all the patients who participated in this study. In addition, the authors want to thank Mrs. Ute Herbst for help with blood collection, Mrs. Alexandra Barhoumi for documentation of obtained blood samples and Mr. Maximilian Brueckl for help with the figures.
Funding: This research was funded by the W. Lutz Stiftung, Nuremberg Germany by an unrestricted grant to WM Brueckl and JH Ficker.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-587
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tlcr-21-587
Peer Review File: Available at https://dx.doi.org/10.21037/tlcr-21-587
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-587). The following authors received honoraria for lectures, presentation, speakers bureaus, manuscript writing or educational events: WMB from AstraZeneca, Boehringer, Novartis, MSD, BMS, Roche and Lilly; JHF from AstraZeneca, Boehringer, Novartis, MSD, BMS, Pfizer, Gilead and Roche; FPMR from Roche, Astra Zeneca and Chugai Pharma; SE from Roche. The following authors received support for attending meetings and/or travel: WMB from Boehringer, AstraZeneca and Roche; FPMR from Roche and Astra Zeneca; JHF from Boehringer and AstraZeneca. The following authors participated on a data safety monitoring board or an advisory board: WMB on AstraZeneca, Boehringer, Novartis, MSD, Lilly, BMS and Roche; JHF on AstraZenca; FPMR on Pfizer and Roche. The following authors receipt equipment, materials, drugs, medical writing, gifts or other services: WMB from Boehringer (for medical writing); JHF from Novartis (for medical writing); The following authors received consulting fees: AR from AbbVie, AstraZeneca, BMS, Boehringer, Lilly, MSD, Novartis, Pfizer and Roche. The following authors have patents planned, issued or pending: WMB, NFB, RMW and EV with Stratifyer. The following authors received support for the present manuscript: RMW and EV as employees to Stratifyer, WMB and JHF with an unrestricted grant from the W. Lutz Stiftung, Nuremberg, Germany. The following authors have stock or stock options related to the manuscript: RMW and SE (Stratifyer). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics committee of the Friedrich-Alexander-University Erlangen-Nuremberg, Germany (numbers: 56_16B and 62_17B). Written informed consent for blood taking, collecting and analyzing for research purpose was obtained from every patient. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J Clin Oncol 2021;39:2339-49. [Crossref] [PubMed]
- Gandara D, Reck M, Moro-Sibilot D, et al. Fast progression in non-small cell lung cancer: results from the randomized phase III OAK study evaluating second-line atezolizumab versus docetaxel. J Immunother Cancer 2021;9:e001882 [Crossref] [PubMed]
- Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer 2020;20:1185. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020;126:260-70. [Crossref] [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Riedl JM, Barth DA, Brueckl WM, et al. C-Reactive Protein (CRP) Levels in Immune Checkpoint Inhibitor Response and Progression in Advanced Non-Small Cell Lung Cancer: A Bi-Center Study. Cancers (Basel) 2020;12:2319. [Crossref] [PubMed]
- Chen S, Li R, Zhang Z, et al. Prognostic value of baseline and change in neutrophil-to-lymphocyte ratio for survival in advanced non-small cell lung cancer patients with poor performance status receiving PD-1 inhibitors. Transl Lung Cancer Res 2021;10:1397-407. [Crossref] [PubMed]
- Han J, Duan J, Bai H, et al. TCR Repertoire Diversity of Peripheral PD-1+CD8+ T Cells Predicts Clinical Outcomes after Immunotherapy in Patients with Non-Small Cell Lung Cancer. Cancer Immunol Res 2020;8:146-54. [Crossref] [PubMed]
- Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017;114:4993-8. [Crossref] [PubMed]
- Kim KH, Cho J, Ku BM, et al. The First-week Proliferative Response of Peripheral Blood PD-1+CD8+ T Cells Predicts the Response to Anti-PD-1 Therapy in Solid Tumors. Clin Cancer Res 2019;25:2144-54. [Crossref] [PubMed]
- Ricciuti B, Jones G, Severgnini M, et al. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J Immunother Cancer 2021;9:e001504 [Crossref] [PubMed]
- Yamauchi T, Hoki T, Oba T, et al. T-cell CX3CR1 expression as a dynamic blood-based biomarker of response to immune checkpoint inhibitors. Nat Commun 2021;12:1402. [Crossref] [PubMed]
- Shin J, Chung JH, Kim SH, et al. Effect of Platinum-Based Chemotherapy on PD-L1 Expression on Tumor Cells in Non-small Cell Lung Cancer. Cancer Res Treat 2019;51:1086-97. [Crossref] [PubMed]
- Liang Y, Yu M, Zhou C, et al. Variation of PD-L1 expression in locally advanced cervical cancer following neoadjuvant chemotherapy. Diagn Pathol 2020;15:67. [Crossref] [PubMed]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101-8. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016;375:1767-78. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]
- Gros A, Parkhurst MR, Tran E, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 2016;22:433-8. [Crossref] [PubMed]
- Eckstein M, Strissel P, Strick R, et al. Cytotoxic T-cell-related gene expression signature predicts improved survival in muscle-invasive urothelial bladder cancer patients after radical cystectomy and adjuvant chemotherapy. J Immunother Cancer 2020;8:e000162 [Crossref] [PubMed]
- Riaz N, Havel JJ, Makarov V, et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 2017;171:934-949.e16. [Crossref] [PubMed]
- Hopkins AC, Yarchoan M, Durham JN, et al. T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight 2018;3:122092 [Crossref] [PubMed]
- Guibert N, Jones G, Beeler JF, et al. Targeted sequencing of plasma cell-free DNA to predict response to PD1 inhibitors in advanced non-small cell lung cancer. Lung Cancer 2019;137:1-6. [Crossref] [PubMed]
- Yang Q, Chen M, Gu J, et al. Novel Biomarkers of Dynamic Blood PD-L1 Expression for Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer Patients. Front Immunol 2021;12:665133 [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:198-211. [Crossref] [PubMed]


