Platelets and their role in cancer evolution and immune system
Introduction
Platelets are the cellular orchestrators of primary hemostasis. Blood platelets are discoid anucleate cellular fragments originating from the cytoplasm of megakaryocytes. Anucleate platelets are found only in mammals. In lower vertebrates, cells involved in hemostasis and blood coagulation are nucleated and known as thrombocytes (1). Platelets have long been known to prevent bleeding upon injury due to their ability to induce coagulation and thrombus formation. Through expression of adhesion molecules and release of their granule contents, they modulate the immune system and preserve vascular integrity.
Hayem in 1877 provided a firm histologic basis for platelets, though he felt they were the origin of red blood cells and referred to them as “hematoblasts”. Bizzozero, in 1882, introduced the term “blood plates” and documented their importance in blood coagulation and in the formation of thrombus (1). Another century of research was needed to demonstrate that platelets display functional powers, working as healers that deliver growth factors and other soothing molecules to help damaged tissue rebuild, cause inflammation and alert the immune cells (1).
The dynamic crosstalk between tumor cells and their microenvironment is increasingly recognized as a key regulator of malignant progression. Indeed, the metastatic potential of tumor cells continues to evolve outside of the primary tumor site, in response to tumor-host interactions in the bloodstream and at the site of metastasis. Platelet-tumor cell interactions and the signaling pathways that these interactions can stimulate have been identified as fundamental determinants of cancer metastasis. Furthermore, circulating tumor cells arrest in microvessels in distant tissues and need to survive in the vessel as well as at the disseminating site in order to develop metastatic foci. Platelets, macrophages and T-regulatory cells are reported to protect the disseminating cancer cells from immune attack and the stress of a hostile environment (2).
In this short review we will summarize the contribution of platelets to tumor cell survival and the development of metastases. Insight from studies pointing to the formation of platelet-tumor cell aggregates in the bloodstream supports the notion that platelets provide cancer cells with an immune escape mechanism by protecting circulating malignant cells from immune-mediated lysis by natural killer (NK) cells. Existing knowledge and further mechanistic studies might suggest platelets and their functions as a new avenue for antimetastatic therapy and anticancer immunotherapies.
Platelets and hemostasis
Circulating platelets are quiescent but in the setting of a vessel injury they become activated by exposure to collagen, the coagulation protease thrombin and other molecules not normally present in blood. Functionally, platelets are complex cells capable of shape change, translational protein production, protein and metabolite release, cell-cell interactions and paracrine regulation. When the vasculature is damaged or in diseased vessels, platelets are able to respond to a great variety of agonists which bind to specific receptors localized on their membrane. The platelet membrane consists of phospholipids and is covered with glycoproteins (GPs) and integrins which are essential for adhesion, activation and aggregation (3). Platelets contain: (I) dense (d-) granules containing platelet agonists such as serotonin and adenosine diphosphate (ADP) that serve to amplify platelet activation; (II) alpha (α-) granules containing proteins that enhance the activation process and participate in coagulation; (III) lysosomal granules containing glycosidases and proteases (4). A wide variety of mobile transmembrane receptors cover the platelet membrane, with some of them being shared by other cell types while others are expressed only on platelets. The major platelet receptors have a role in hemostasis, but it is also increasingly recognized that a range of receptors are involved in other less well-understood platelet functions such as inflammation, tumor growth, metastasis, or immunological host defense.
When the vasculature is damaged or in diseased vessels, a series of events coordinated both in time and in place are required leading to: (I) platelet arrest onto the exposed sub-endothelium creating a monolayer of activated cells (initiation phase or adhesion); (II) recruitment and activation of additional platelets through the local release of major platelet agonists (extension phase or activation); (III) stabilization of the platelet plug preventing premature disaggregation until wound healing occurs (stabilization phase or aggregation) (3) (Table 1 and Figure 1).
Full table
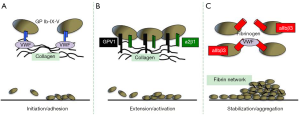
Thrombus formation in response to tissue trauma initiates the interaction of platelets with the extracellular matrix components exposed to blood, particularly von Willebrand factor (VWF) and collagen (5). The GPIb/IX/V complex is the major platelet receptor mediating interaction with VWF and collagen (Figure 1). The next step required for thrombus formation is the recruitment of additional platelets from the flowing blood, in a process commonly referred to as extension or platelet activation (Figure 1). This is made possible by the local accumulation of platelet-secreted agonists ADP and thromboxane A2, epinephrine and thrombin (6,7). Two receptors on the platelet surface bind directly to collagen, the GPVI immunoglobulin superfamily member and the integrin a2β1 commonly referred to as GPIa/IIa, VLA-2 or CD49b/CD29 (Figure 1). GPVI has two extracellular immunoglobulin domains, a mucin-like core, a short peptide linker sequence, a transmembrane domain and a short cytoplasmic tail that binds Fyn and Lyn Src kinases. GPVI is also constitutively complexed with the low affinity immunoglobulin ITAM (immunoreceptor tyrosine-based activation motif) receptor FcγRIIa, which signals through a single ITAM in its cytosolic domain and is a critical mediator of platelet activation in immune thrombocytopenia (8-10), heparin-induced thrombocytopenia (11), bacterial infection (12,13) and cancer (14). The a2β1 integrin also plays a role in the adhesion of platelets to collagen and subsequent optimal activation (Figure 1). G protein-coupled receptors (GPCRs) are seven-transmembrane spanning signaling molecules that play crucial roles in the extension of the platelet plug by most soluble platelet agonists. GPCRs are associated with a group of G proteins consisting of three subunits termed alpha, beta, and gamma and are classified into four families according to their α subunit; Gαi, Gαs, Gα12/13, and Gαq (15). Two classes of GPCR, the purinoceptor 2 (P2) receptors, P2Y1 and P2Y12 are required for optimal ADP-induced aggregation and ADP-promoted thrombus growth (16). Thrombin that is rapidly generated at sites of vascular injury plays a major role in promoting and stabilizing thrombi (17). The thrombin-induced platelet responses are mediated mainly by two protease activated receptors (PAR), PAR-1 and PAR-4 (Table 1).
The last phase in the formation of an effective thrombus at the site of vascular injury is known as stabilization or aggregation (Figure 1). The most relevant contact-dependent signaling events during this stabilization phase occur through integrins, particularly aIIbβ3 (also known as GPIIb-IIIa). aIIbβ3 causes a conformational change that enables it to bind fibrinogen and VWF, allowing stable bridges between platelets (Figure 1). Besides integrins, platelets express junctional adhesion molecules which support cohesive and signaling interactions between adjacent platelets and between platelets and leukocytes favoring thrombus stabilization (18). The formation of a fibrin network upon activation of the coagulation cascade is generally considered the last critical event contributing to thrombus stability (Figure 1). Fibrin formation depends on the monocyte-derived tissue factor (TF) carried out by microparticles, with minimal contribution of vessel wall TF. These microparticles are captured by the thrombus through the interaction between P-selectin expressed on the surface of activated platelets and PSGL-1 present on the microparticles (19) (Table 1).
The role of C-type lectin-like receptor 2 (CLEC-2), a type II transmembrane protein that signals via a single YxxL sequence known as a hemITAM, remains to be better defined (20). CLEC-2 is the receptor for the type I transmembrane GP podoplanin, which is widely expressed outside of the vasculature, including lymphatic endothelial cells, type I lung alveolar cells, lymph node stromal cells and the choroid plexus epithelium. Podoplanin is also present on inflammatory macrophages (21,22) and on a subset of activated T-helper (Th) 17 cells (23,24). The function of CLEC-2 in hemostasis is not clear with reports indicating that it either plays a minor role (25,26) or that it plays no role (27). However, the bidirectional relationship between CLEC-2 and podoplanin has been described and considered in the context of tumor growth and metastasis (28).
The role of platelets in cancer evolution
The association of platelet clotting properties and overt or occult malignancy was reported in the 19th century. Gasic et al., first described the association between platelet number and metastatic cancer potential (29). They reported the finding that neuraminidase in vivo produces thrombocytopenia, with a close correlation between the decrease in platelet levels and the decrease in metastases (29). Thrombocytopenia experimentally induced by a variety of mechanisms has also been associated with a reduction in the number of metastases in tumor transplant models (29,30). Often numbering over 3-4 trillion in an individual patient with cancer, elevated platelet numbers have been associated with tumor metastasis and poor prognosis (31). Tumor cells can aggregate and activate platelets, leading to the initiation of a thrombus through the process known as tumor cell-induced platelet aggregation (TCIPA) (32). TCIPA correlates with both the tendency to thrombosis induced by tumors and, reciprocally, to their metastatic potential.
But how do platelets help tumor cells to survive in the blood environment and finally exit the bloodstream? One way is that platelets protect metastatic tumor cells from immune surveillance as they travel round the circulatory system and help tumor cells to attach to endothelium upon their arrest at metastatic sites (33). Platelet and endothelial cell adhesion proteins may also facilitate metastasis by augmenting tumor cell extravasation, a hypothesis supported by the observation that tumor metastases are reduced in mice lacking the vascular adhesion molecules P- and L-selectin (34). Vascular endothelial growth factor (VEGF), an α-granule constituent released by activated platelets as a pro-angiogenic protein, promotes vasculogenesis by stimulating endothelial migration and proliferation (35). Indeed, platelets provide a major serum source of many pro-angiogenic proteins in the circulation of patients with cancer (36). In addition, platelets take up tumor-derived secreted membrane vesicles that can contain tumor-associated RNA and can serve as a potential biomarker source for cancer diagnostics (37).
Genetic studies have demonstrated that platelets promote extravasation of colon and breast cancer cells by inducing epithelial-mesenchymal-like transition through secretion of transforming growth factor β and through direct contact with cancer cells (38). Schumacher and colleagues found that adenine nucleotides released from tumor cell-activated platelets induce opening of the endothelial barrier to allow trans-endothelial migration of tumor cells (39). Genetic ablation of P2Y2, expressed in endothelial cells, prevents vascular leakage and suppresses tumor extravasation (39). Reduced expression of P2Y2 in endothelial cells suppresses platelet-dependent tumor extravasation in vitro, indicating that platelets lose the endothelial barrier by releasing ATP-containing granules, which enhances extravasation of cancer cells (39).
Labelle et al., reported that platelets and granulocytes are sequentially recruited to disseminated tumor cells to form “early metastatic niches” that promote metastatic progression. Platelet-derived CXCL5/7 chemokines and not tumor cell-derived signals recruit granulocytes and guide the formation of early metastatic niches, which are crucial for metastasis (40). The sialoglycoprotein Aggrus/podoplanin expressed by tumor cells activates platelets through CLEC-2 and, reciprocally, the system promotes tumor growth and metastasis in experimental models (41,42). Interestingly podoplanin-positive cancer-associated fibroblasts play an important role in primary resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) and may be an ideal therapeutic target for use in combination therapy with EGFR-TKIs in non-small cell lung cancer (NSCLC) patients with EGFR mutations (43).
Platelets and immune system
The microenvironment within the bloodstream is hostile to circulating tumor cells and their survival before extravasation is crucial for metastasis (Figure 2). Data from mouse models suggest that the recruitment of immunosuppressive cells, like macrophages, neutrophils and platelets, to tumor cells protects metastatic cancer cells from surveillance by killer cells, which nullifies the effects of immunotherapy and thus enables metastasis (44). Antibody-induced (29) or genetic (45) depletion of platelets inhibits metastasis, whereas platelet reconstitution restores metastatic activity (46), as seen in several independent mouse models. Furthermore, platelets arrest tumor cells in capillaries and provide a survival signal, which in turn allows a chemotactic gradient of CC-chemokine ligand 2 (CCL2) to be established to recruit inflammatory monocytes. These monocytes differentiate into metastasis-associated macrophages (MAMs) that promote extravasation and cancer cell survival through cell-to-cell contact (47,48).
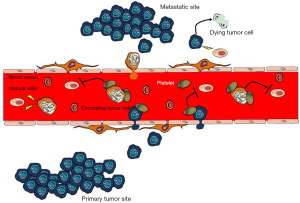
Nieswandt et al., were among the first to report that the main contribution of platelets to metastasis seems to consist in protection of tumor cells from NK cell lysis (33) (Figure 2). Only very few circulating tumor cells establish metastatic foci in patients with cancer despite thousands of cells being released into the circulatory system every day (49). Thus, the metastatic steps following tumor cell survival, extravasation and metastatic growth are rate-limiting processes for metastasis. Macrophages, neutrophils and platelets have been reported to promote these inefficient steps of metastasis (44). There are data indicating that platelet activation and the resultant fibrin clot formation promote the early survival of cancer cells that are lodged at the metastatic sites by shielding them from NK cells (Figure 2). For instance, intravenously injected melanoma cells develop fewer metastatic foci in the lungs of platelet-depleted mice than in normal mice (45). Moreover, genetic loss of Gαq, prothrombin or fibrinogen reduces the number of residual cancer cells in the lungs 24 hours after intravenous injection (45). The precipitous loss of embolus cancer cells in these gene-targeted mice is rescued by NK cell ablation (50,51). Metastasis is nearly completely inhibited in mice that lack platelets based on nuclear factor erythroid-derived 2 (NFE2) knockout, which interrupts platelet production by interfering with megakaryocyte maturation (45). These results, together with reduced metastasis in PAR4-knockout mice and fibrinogen-knockout mice, indicate that platelets and their activation have an essential role in hematogenous tumor cell spreading and that this process involves thrombin-dependent and thrombin-independent mechanisms (45). Platelets of PAR4-knockout mice are unable to aggregate in response to thrombin, but their thromboxane and GPVI receptors are not disturbed (45,52). The thrombin inhibitor hiruidin further reduces metastasis in PAR4-knockout mice, implying a mechanism that involves other thrombin ligands, such as fibrinogen (53) or PARs expressed by the tumor cells. Mice with PAR1 and PAR2 deficiency, in which endothelial responses to coagulation proteases—but not those of the experimentally introduced tumor cells—are attenuated, were not protected against metastasis (45). Thrombin activates PAR1 and PAR2 receptors that are expressed by host and tumor cells, and supports tumor growth, angiogenesis and metastasis (54,55).
The exact mechanisms of how platelet activation and fibrin clotting protect from NK cells still need to be defined. Platelets may provide a physical barrier to NK cell contact, and at the same time exert paracrine suppression of NK-mediated cytolytic activity (56). For instance, transforming growth factor-β from platelets diminishes NK granule mobilization, cytotoxicity and interferon-γ secretion (57). Other soluble mediators including prostaglandin E2 (PGE2), may have similar activity (56). Overall, the ability of platelets to induce the formation of hetero-aggregates between tumor cells, platelets and leukocytes may play an important part in determining tumor cell survival within the microvasculature of target organs of metastasis.
Conclusions
It is important to clearly identify the factors released by platelets that promote cancer metastasis and stimulate antitumor immune responses. Employing specific inhibitors of platelet function or neutralizing agents would enable the development of new treatments without the need for lowering platelet count. Such anti-platelet treatment combined with chemotherapy or immunotherapy would enhance delivery to the tumor and may represent a significant improvement in our ability to fight cancer with minimal side effects and without increasing the risk of bleeding.
Acknowledgements
Funding: This work was supported by grants from the La Caixa Foundation and Red Tematica de Investigacion Cooperativa en Cancer (RTICC; grant RD12/0036/0072), by a Fellowship Award of the International Association for the Study of Lung Cancer (IASLC) and a grant of the Italian Association for Cancer Research (AIRC My First AIRC grant No. 14282).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science 2010;328:562-4. [PubMed]
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011;11:123-34. [PubMed]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237-49. [PubMed]
- Reed GL, Fitzgerald ML, Polgár J. Molecular mechanisms of platelet exocytosis: insights into the "secrete" life of thrombocytes. Blood 2000;96:3334-42. [PubMed]
- Ma YQ, Qin J, Plow EF. Platelet integrin alpha(IIb)beta(3): activation mechanisms. J Thromb Haemost 2007;5:1345-52. [PubMed]
- Broos K, Feys HB, De Meyer SF, et al. Platelets at work in primary hemostasis. Blood Rev 2011;25:155-67. [PubMed]
- Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med 2011;17:1423-36. [PubMed]
- McKenzie SE, Taylor SM, Malladi P, et al. The role of the human Fc receptor Fc gamma RIIA in the immune clearance of platelets: a transgenic mouse model. J Immunol 1999;162:4311-8. [PubMed]
- Stolla M, Stefanini L, André P, et al. CalDAG-GEFI deficiency protects mice in a novel model of Fcgamma RIIA-mediated thrombosis and thrombocytopenia. Blood 2011;118:1113-20. [PubMed]
- Urbanus RT, van der Wal DE, Koekman CA, et al. Patient autoantibodies induce platelet destruction signals via raft-associated glycoprotein Ibalpha and Fc RIIa in immune thrombocytopenia. Haematologica 2013;98:e70-2. [PubMed]
- Warkentin TE, Greinacher A, Gruel Y, et al. Laboratory testing for heparin-induced thrombocytopenia: a conceptual framework and implications for diagnosis. J Thromb Haemost 2011;9:2498-500. [PubMed]
- Tilley DO, Arman M, Smolenski A, et al. Glycoprotein Ibalpha and FcgammaRIIa play key roles in platelet activation by the colonizing bacterium, Streptococcus oralis. J Thromb Haemost 2013;11:941-50. [PubMed]
- Arman M, Krauel K, Tilley DO, et al. Amplification of bacteria-induced platelet activation is triggered by FcgammaRIIA, integrin alphaIIbbeta3, and platelet factor 4. Blood 2014;123:3166-74. [PubMed]
- Mitrugno A, Williams D, Kerrigan SW, et al. A novel and essential role for FcgammaRIIa in cancer cell-induced platelet activation. Blood 2014;123:249-60. [PubMed]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science 2002;296:1636-9. [PubMed]
- Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res 2006;99:1293-304. [PubMed]
- Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost 2003;1:1602-12. [PubMed]
- Brass LF, Zhu L, Stalker TJ. Novel therapeutic targets at the platelet vascular interface. Arterioscler Thromb Vasc Biol 2008;28:s43-50. [PubMed]
- Vandendries ER, Furie BC, Furie B. Role of P-selectin and PSGL-1 in coagulation and thrombosis. Thromb Haemost 2004;92:459-66. [PubMed]
- Gitz E, Pollitt AY, Gitz-Francois JJ, et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood 2014;124:2262-70. [PubMed]
- Hou TZ, Bystrom J, Sherlock JP, et al. A distinct subset of podoplanin (gp38) expressing F4/80+ macrophages mediate phagocytosis and are induced following zymosan peritonitis. FEBS Lett 2010;584:3955-61. [PubMed]
- Kerrigan AM, Navarro-Nuñez L, Pyz E, et al. Podoplanin-expressing inflammatory macrophages activate murine platelets via CLEC-2. J Thromb Haemost 2012;10:484-6. [PubMed]
- Peters A, Pitcher LA, Sullivan JM, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 2011;35:986-96. [PubMed]
- Miyamoto Y, Uga H, Tanaka S, et al. Podoplanin is an inflammatory protein upregulated in Th17 cells in SKG arthritic joints. Mol Immunol 2013;54:199-207. [PubMed]
- May F, Hagedorn I, Pleines I, et al. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood 2009;114:3464-72. [PubMed]
- Suzuki-Inoue K. Essential in vivo roles of the platelet activation receptor CLEC-2 in tumour metastasis, lymphangiogenesis and thrombus formation. J Biochem 2011;150:127-32. [PubMed]
- Bender M, May F, Lorenz V, et al. Combined in vivo depletion of glycoprotein VI and C-type lectin-like receptor 2 severely compromises hemostasis and abrogates arterial thrombosis in mice. Arterioscler Thromb Vasc Biol 2013;33:926-34. [PubMed]
- Lowe KL, Navarro-Nunez L, Watson SP. Platelet CLEC-2 and podoplanin in cancer metastasis. Thromb Res 2012;129 Suppl 1:S30-7. [PubMed]
- Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A 1968;61:46-52. [PubMed]
- Karpatkin S, Pearlstein E. Role of platelets in tumor cell metastases. Ann Intern Med 1981;95:636-41. [PubMed]
- Erpenbeck L, Schön MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood 2010;115:3427-36. [PubMed]
- Bastida E, Almirall L, Ordinas A. Tumor-cell-induced platelet aggregation is a glycoprotein-dependent and lipoxygenase-associated process. Int J Cancer 1987;39:760-3. [PubMed]
- Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 1999;59:1295-300. [PubMed]
- Wahrenbrock M, Borsig L, Le D, et al. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest 2003;112:853-62. [PubMed]
- Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585-601. [PubMed]
- Kuznetsov HS, Marsh T, Markens BA, et al. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells. Cancer Discov 2012;2:1150-65. [PubMed]
- Nilsson RJ, Balaj L, Hulleman E, et al. Blood platelets contain tumor-derived RNA biomarkers. Blood 2011;118:3680-3. [PubMed]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011;20:576-90. [PubMed]
- Schumacher D, Strilic B, Sivaraj KK, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013;24:130-7. [PubMed]
- Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A 2014;111:E3053-61. [PubMed]
- Takagi S, Sato S, Oh-hara T, et al. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS One 2013;8:e73609. [PubMed]
- Goubran HA, Stakiw J, Radosevic M, et al. Platelets effects on tumor growth. Semin Oncol 2014;41:359-69. [PubMed]
- Yoshida T, Ishii G, Goto K, et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin Cancer Res 2015;21:642-51. [PubMed]
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015;15:73-86. [PubMed]
- Camerer E, Qazi AA, Duong DN, et al. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 2004;104:397-401. [PubMed]
- Karpatkin S, Pearlstein E, Ambrogio C, et al. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest 1988;81:1012-9. [PubMed]
- Gil-Bernabé AM, Ferjancic S, Tlalka M, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 2012;119:3164-75. [PubMed]
- Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011;475:222-5. [PubMed]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009;9:239-52. [PubMed]
- Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005;105:178-85. [PubMed]
- Palumbo JS, Talmage KE, Massari JV, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood 2007;110:133-41. [PubMed]
- Sambrano GR, Weiss EJ, Zheng YW, et al. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature 2001;413:74-8. [PubMed]
- Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000;96:3302-9. [PubMed]
- Versteeg HH, Schaffner F, Kerver M, et al. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer Res 2008;68:7219-27. [PubMed]
- Hu L, Roth JM, Brooks P, et al. Twist is required for thrombin-induced tumor angiogenesis and growth. Cancer Res 2008;68:4296-302. [PubMed]
- Palumbo JS, Degen JL. Mechanisms linking tumor cell-associated procoagulant function to tumor metastasis. Thromb Res 2007;120 Suppl 2:S22-8. [PubMed]
- Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 2009;69:7775-83. [PubMed]


