Immunotherapy meets targeted therapy: will this team end the war against cancer?
Introduction
Cancer remains one of the main causes of morbidity and mortality worldwide; with lung cancer as the leading cause of cancer-related death for the last years (1,2). Targeted therapies have become part of our daily armamentarium against lung and other types of cancer. A new era since the development of immunotherapy has arrived, where the most widely studied scenario is melanoma. Most of the works mentioned in this review relate to this particular disease; but time has shown that those finding most of the time can be adapted to lung cancer (3).
Immunotherapy
The immune system recruitment may represent a powerful and innovative strategy in cancer therapy; cancer immunotherapy is likely to become a fundamental part of modern oncology. The number of clinical trials involving immunotherapy has skyrocketed. As previously mentioned, its main goal is to suppress the mechanisms of resistance of cancer cells to tyrosine kinase inhibitors (TKIs) restore the availability of antigens to cytotoxic T lymphocytes and antigen presenting cells (APCs) and to restore the function of these cells and T lymphocytes (4).
The first effective immunotherapies approved by the US Food and Drug Administration in melanoma included interleukin-2 for metastatic disease and interferon alpha in the adjuvant setting. These were followed by a group of new therapies, including checkpoint-blocking antibodies targeting cytotoxic T lymphocyte-associated protein 4 and programmed cell death protein 1 due to their relevance in the maintenance of peripheral immune tolerance (5).
One of the new standards of care in melanoma—Ipilimumab—showed enhanced overall survival with durable responses, lasting even longer than 30 months in about 20% of the treated population (6,7).
However, its combination with other antibodies such as Nivolumab has shown to have complementary activity in metastatic melanoma. This combination resulted in significantly longer progression-free survival than ipilimumab alone (8,9).
Nivolumab and Pembrolizumab are two FDA-approved monoclonal antibodies that block the programmed death-1 receptor (PD-1, CD279), resulting in dis-inhibition of tumor-specific immune responses. They show highly durable response rates and long-term safety, validating the importance of the programmed cell death protein 1 pathway blockade for treatment of several malignances (10,11).
Although drug-related adverse events have been reported in a majority of patients receiving either Pembrolizumab or Nivolumab, only 8% to 15% experienced significant side effects (grade 3–5). Serious immune related events such as dermatitis, diarrhea/colitis, hepatitis, and pancreatitis have been reported in relatively few patients (up to 2%) (12-14).
A glance at targeted therapy
Targeted therapies exert their activity by blocking an essential mutant protein or pathway required for tumor survival and growth (15). These treatments had become familiar assets in our daily practice. We all have seen their effects, the striking regressions in some patients defined by their molecular profiles (i.e., EGFR mutated and EML4-ALK translocated patients); nevertheless, such impressive responses are followed by progressive disease due to drug-resistance variants in most cases (16-18).
Rationale for the combination of both strategies
By looking at the weaknesses and strengths of both approaches, it seems that their combination may be synergistic against cancer. Among the properties of targeted therapies that enhance immunotherapy are promotion of dendritic cell maturation; trigger of both activation and differentiation of memory T-cells; an increase in the expression of death receptors and a diminution of survival signals that sensitizes malignant cells to immune-mediated death (Figure 1).
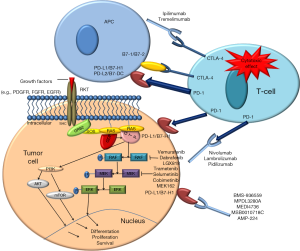
Not only are the responses to the combination of these approaches under evaluation; but is also of great interest to find the appropriate dose, timing and best sequence of them in order to achieve successful results. Not to forget that the intensification of the response might lead to higher toxicity.
Moreover, the oncogene addiction held by malignant tumors is broken by targeted therapies; this effect may potentiate the immune response against the tumor and thus facilitate tumor clearance by T-cells (19,20).
Conclusions
Decades of research are now leading to what seems the ultimate strategy against cancer. The understanding of the molecular pathways involved in the development of malignancies and the discovery of specific targets and targeted therapies against them; as well as the widespread understanding of the role of immunotherapy in cancer has led to a progress in the development of new treatments. The gap between “bench and bed” is getting smaller. The combination of both strategies seems to be synergistic and in the future, we will know if it truly leads to the awaited cure. Many clinical trials are ongoing whose results are highly expected.
Acknowledgements
D Morales-Espinosa’s work is supported with grants from IASLC’s Lung Cancer Research Fellowship Award and ESMO’s Translational Research Fellowship Award. Work in R. Rosell’s Laboratory is partially supported by a grant from Fundació La Caixa.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Globocan, fact sheets by cancer. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Pennell NA. Understanding the Rationale for Immunotherapy in Non-Small Cell Lung Cancer. Semin Oncol 2015;42 Suppl 2:S3-S10. [PubMed]
- Talmadge JE. Development of immunotherapeutic strategies for the treatment of malignant neoplasms. Biotherapy 1992;4:215-36. [PubMed]
- Snyder A, Zamarin D, Wolchok JD. Immunotherapy of Melanoma. Prog Tumor Res 2015;42:22-9. [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [PubMed]
- Somasundaram R, Herlyn M. Nivolumab in combination with ipilimumab for the treatment of melanoma. Expert Rev Anticancer Ther 2015;15:1135-41. [PubMed]
- Wolchok JD. PD-1 Blockers. Cell 2015;162:937. [PubMed]
- Faghfuri E, Faramarzi MA, Nikfar S, et al. Nivolumab and pembrolizumab as immune-modulating monoclonal antibodies targeting the PD-1 receptor to treat melanoma. Expert Rev Anticancer Ther 2015;15:981-93. [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [PubMed]
- Druker BJ, David A. Karnofsky Award lecture. Imatinib as a paradigm of targeted therapies. J Clin Oncol 2003;21:239s-245s. [PubMed]
- Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001-10. [PubMed]
- Morales-Espinosa D, García-Román S, Karachaliou N, et al. Pharmacogenomics in the treatment of lung cancer: an update. Pharmacogenomics 2015;16:1751-60. [PubMed]
- Rosell R, Karachaliou N, Morales-Espinosa D, et al. Adaptive resistance to targeted therapies in cancer. Transl Lung Cancer Res 2013;2:152-9. [PubMed]
- Chiarle R, Martinengo C, Mastini C, et al. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat Med 2008;14:676-80. [PubMed]
- Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer 2012;130:1948-59. [PubMed]


