
Tagrisso incremental therapy in a case of meningeal metastasis of lung cancer with EGFR mutation: a case report
Introduction
The central nervous system (CNS) is a common site of metastasis in patients with non-small cell lung cancer (NSCLC). The proportion of NSCLC patients with brain metastases or meningeal metastases during disease development ranges from 23% to 36% (1). With the development of individualized treatment and the widespread use of targeted therapy with tyrosine kinase inhibitors (TKIs), the median survival of patients with NSCLC is 16 months, while that of NSLC patients with meningeal metastases is only 4.5 months (2). Persistent dizziness often accompanies meningeal metastases, headaches, severe vomiting, hallucinations, and mental disorders, which cause considerable pain and discomfort. Currently, diagnosing meningeal metastasis is often challenging due to the generally poor condition of patients or the low sensitivity of diagnostic techniques.
Meanwhile, treatment methods for meningeal metastasis are limited. Here, we report a patient with stage IV NSCLC with epidermal growth factor receptor (EGFR) 21 exon L858R mutation, with EGFR 20 exon T790M mutation detected after the failure of treatment with Tarceva. The patient was subsequently confirmed as meningeal metastasis by cerebrospinal fluid (CSF) cytology during therapy with Tagrisso (80 mg qd). After next-generation sequencing (NGS) of the patient’s CSF confirmed that the EGFR mutation type had not changed, we deduced that the cause of meningeal metastasis was an insufficient concentration of TKIs in the CSF. Therefore, we suggested treating with a double dose of Tagrisso (160 mg qd), which the patient accepted. A double dose of Tagrisso is rare in clinical experience. Thanks to this treatment, the patient’s progression-free survival (PFS) was extended by 7 months, and he had an overall survival (OS) of more than 5 years, which is unusual in the clinic. We hope this case report will provide more clues for the diagnosis and treatment of such patients.
We present the following article in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-451/rc).
Case presentation
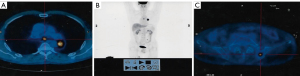
A 65-year-old male was admitted to the hospital in February 2014 after a routine physical examination revealed elevated CEA (193 ng/mL). No other clinical symptoms, including cough or chest pain, were observed. The patient had a history of smoking 900 cigarettes a year. He had no history of hypertension, diabetes, exposure to poisons or chemicals, or regular medication, and no significant family history. Physical examination (PE) of the patient’s lungs detected no crackles or wheezes, and a neurological examination revealed no abnormalities at that time. Systemic positron emission tomography/computed tomography (PET/CT) on 28th February 2014 showed malignant lesions of the dorsal segment of the left lower lung with metastasis of the subcarinal lymph nodes and left ilium (Figure 1). Brain magnetic resonance imaging (MRI) on 2nd March 2014 showed bilateral frontoparietal ischemic foci. Bronchoscopy revealed swelling of the mucosa in the dorsal segment of the left lower lung and occlusion of the orifice, and biopsy on 6th March 2014 showed adenocarcinoma with EGFR 21 exon L858R mutation (Figure 2). The patient, a retired employee with national medical insurance, was not religious and actively cooperated during diagnosis. The patient was diagnosed with stage IV adenocarcinoma of the left lung with EGFR 21 exon L858R mutation and a bone metastasis based on PE, imaging, and laboratory results.
The patient did not indicate radical surgery or radiotherapy as the first-line treatment. Therefore, chemotherapy and self-management therapy were chosen, and the patient accepted a 4-cycle chemotherapy regimen (pemetrexed 500 mg/m2 plus cisplatin 75 mg/m2, 1 cycle every 28 days) from 7th March 2014 to 23rd May 2014 (Tables 1,2). He also received adequate home care. According to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1), the pulmonary lesions achieved partial response (PR). The patient showed good tolerance to chemotherapy with a transient gastrointestinal response. The patient felt well and had only a light cough. Based on the patient’s imaging results and clinical symptoms, we considered chemotherapy the first-line treatment was effective. The patient’s confidence increased significantly, and he agreed with our recommendation, based on the results of the SATURN study, to take Tarceva (150 mg qd) as maintenance therapy, starting on 15th June 2014. The patient developed a grade I rash (according to the Common Terminology Criteria for Adverse Events, CTCAE), which was tolerable, and his condition remained as PR.
Table 1
| Relevant past medical history and interventions |
| Smoking history of 900 cigarettes a year |
| No personal history of diseases such as hypertension or diabetes |
| No psychosocial history |
| No exposure to poisons or chemicals |
| No regular medication |
| No significant family history |
Table 2
| Dates | Initial and follow-up visits | Diagnostic testing (including dates) | Interventions |
|---|---|---|---|
| Feb 2014 | Physical examination revealed elevated CEA without clinical symptoms | PET/CT: malignant lesions of the dorsal segment of the left lower lung with metastasis of the subcarinal lymph nodes and left ilium (28th February 2014) | 4 cycles of chemotherapy (pemetrexed plus cisplatin); Tarceva 150 mg qd given as maintenance therapy |
| Brain MRI: bilateral frontoparietal ischemic foci (2nd Mar 2014) | |||
| Bronchoscopy: swelling of the mucosa in the dorsal segment of the left lower lung, occlusion of the orifice, biopsy showing adenocarcinoma with EGFR 21 exon L858R mutation (6th March 2014) | |||
| Jun 2016 | The patient complained of asthenia and light cough | CT: local progression in the chest lesion (20th June 2016) | Tarceva treatment in combination with local radiotherapy |
| Dec 2016 | Patient complained of pain in the buttocks and caudal vertebrae | Bone scan: new lesion of pelvic metastasis | Tarceva treatment in combination with sacroiliac joint and ilium radiotherapy |
| Aug 2017 | Patient complained of progressive cough and hip pain | CT scan and bone scan: indicated lung and bone progression again (18th August 2017) | Tarceva treatment stopped, and patient given Tagrisso 80 mg qd |
| Blood NGS test: EGFR 21 exon L858R mutation combined with EGFR 20 exon T790M mutation (25th August 2017) | |||
| Dec 2018 | Patient complained of cough, shortness of breath, and obvious headache with disturbance of consciousness | CT scan: the target lesion of the left lower lung was enlarged, and the pleural effusion had increased (19th December 2018) | Tagrisso dose doubled from 80 mg qd to 160 mg qd |
| Brain MRI: lacunar infarction (25th Dec 2018) | |||
| CSF cytology: adenocarcinoma cells (26th December 2018) | |||
| CSF NGS: EGFR 21 exon L858R mutation combined with EGFR 20 exon T790M mutation (5th January 2019) | |||
| Aug 2019 | Headaches and unconsciousness reoccurred | None | Best support treatment and hospice care |
CEA, carcinoembryonic antigen; CT, computed tomography; CSF, cerebrospinal fluid; EGFR, epidermal growth factor receptor; MRI, magnetic resonance imaging; NGS, next-generation sequencing; PET, positron emission tomography.
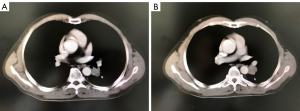
The patient’s first sign of progressive disease (PD) came on 20th June 2016, when he complained of asthma and a light cough. A CT scan indicated local progression of the chest lesion (PFS 1 =24 months) (Figure 3). Because the disease had only progressed in the lungs, we believed the patient would benefit from Tarceva, so we suggested he receive Tarceva treatment in combination with local radiotherapy (treatment with Tarceva had been suspended during radiotherapy). The patient saw no significant increase in his clinical symptoms, and accepted radiotherapy for the left lower lung lesions and left hilar lymph nodes (55 Gy/22 Fx) (Figure 4; Tables 1,2). According to RECIST 1.1, the treatment effect was evaluated as PR.
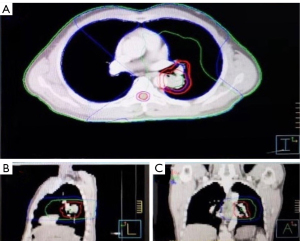
In December 2016, the patient complained of pain in the buttocks and caudal vertebrae (Numeric Rating Scale: 8), and a bone scan indicated PD of bone metastasis (PFS 2 =6 months) (Figure 5). Due to the local progression, sacroiliac joint and ilium radiotherapy (30 Gy/10 Fx) from 26th December 2016, in combination with Tarceva (150 mg qd), was suggested (Tables 1,2). After radiotherapy, the patient’s pain was significantly relieved. The patient had full compliance due to the reduction of his clinical symptoms.
In August 2017, the patient returned to the hospital again, complaining of progressive cough and hip pain. The third PD was confirmed by chest CT scan and bone scan on 18th August 2017, which indicated disease progression in the lung and bone again (PFS 3 =8 months). Multiple processes and 38 months of Tarceva administration forced us to consider the possibility of TKI resistance. We suggested a second biopsy, and the patient agreed to undergo a blood NGS test. The results from 25th August 2017 showed EGFR 21 exon L858R mutation combined with EGFR 20 exon T790M mutation. Based on the AURA3 results, the patient started Tagrisso (80 mg qd) orally on 26th August 2017 (Tables 1,2). One month later, an imaging examination showed the treatment effect was PR. The patient’s clinical symptoms had improved; the grade I rash had disappeared, liver function was normal, and no diarrhea.
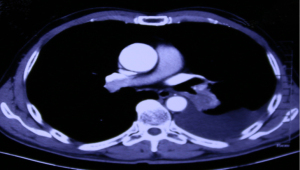
The fourth PD happened in December 2018. The patient had experienced cough, shortness of breath, and obvious headache with impaired consciousness (PS score was 3). On 19th December 2018, a CT scan indicated that the target lesion in the left lower lung had grown, and the pleural effusion had increased (Figure 6). Brain MRI on 25th December 2018 showed only lacunar infarction. CNS metastasis was highly suspected, and on 26th December 2018, the patient was convinced to undergo CSF cytology, which found adenocarcinoma cells (PFS 4 =16 months). On 5th January 2019, NGS of CSF detected EGFR 21 exon L858R mutation combined with EGFR 20 exon T790M mutation. The patient’s diagnosis was updated as stage IV adenocarcinoma of the left lung with bone and meningeal metastasis. Through the CNS symptoms, the patient felt extremely anxious and irritable. After sufficient communication with the patient, he agreed to increase the dose of Tagrisso from 80 to 160 mg qd (Tables 1,2). After receiving a double dose of Tagrisso, the patient’s neurological symptoms gradually disappeared, the chest condition was SD, and none of the patient’s side effects were aggravated. Tagrisso incremental treatment continued until progression on 14th August 2019, when CNS-related symptoms reappeared (PFS 5 =7 months). The patient chose palliative care at home (Tables 1,2).
During the 5-year treatment period, the patient adopted the best self-care and went to the outpatient clinic monthly for further consultation. Any changes in medication and symptoms were recorded, and there were no uncontrolled adverse events.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional review board of Shanghai Chest Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In the past decade, the prolonged survival time of lung cancer patients has seen the probability of CNS metastasis in patients with NSCLC increase significantly. According to the latest statistics, meningeal metastasis among patients with NSCLC is 3.4–3.8%, and the incidence among patients with EGFR mutation is as high as 9.4% (3). This phenomenon may be related to the longer survival time of patients with EGFR mutations, and the inability of EGFR TKI drugs to penetrate the blood-brain barrier, which leads to lower concentrations of TKIs in the CSF (4,5). At present, the clinical diagnosis of meningeal metastases is generally difficult, and treatment options are lacking. According to the diagnosis and treatment of this case, we will now present some innovative ideas for diagnosing and treating meningeal metastases for clinical reference.
Diagnosis of meningeal metastasis
According to National Comprehensive Cancer Network guidelines, meningeal metastasis is currently diagnosed based on a combination of clinical CNS-related symptoms, imaging evidence (MRI), and the detection of tumor cells in CSF. The patient here experienced headache and unconsciousness during treatment with Tagrisso (80 mg po. qd); however, brain MRI suggested lacunar infarction without CNS metastases. The meningeal metastases were highly suspected, considering the patient’s medical history. We recommend the patient undergoes CSF cytology to confirm. The patient accepted this recommendation, and adenocarcinoma cells were found in CSF, confirming lung cancer meningeal metastasis diagnosis. Currently, the sensitivity of MRI for diagnosing meningeal metastasis is 53% (6), while the sensitivity of CSF for detecting tumor cells is only 50% (7,8), meaning some meningeal metastasis patients are unable to obtain a definite diagnosis. This situation urgently needs to be improved. Some studies have shown that circulating tumor DNA (ctDNA) content in CSF is significantly higher than that in plasma. The sensitivity of CSF circulating tumor cells (CSF CTCs) detection is 95.2% (9), but it can become a new diagnostic standard that requires more medical evidence and real-world data.
CSF NGS testing
Tumor metastasis is a complex multi-step continuous process. Whole exome sequencing (WES) sequencing was used to study the evolutionary trajectory of lung cancer by analyzing the gene cloning between primary and metastatic (P-M) lesions. The results suggested that metastasis is a process of tumor evolution with temporal and spatial genomic heterogeneity (10,11) and has target organ specificity (12). Multiple tumor mutations can be detected in CSF ctDNA using the NGS method in patients with meningeal metastasis. The detection rate of gene mutations in CSF is highly consistent with the primary tumor mutation rate (89.5%) (9,13), which is superior to peripheral blood detection, suggesting that CSF CTCs may be effective liquid biopsy specimens.
However, the NGS detection mechanism of CSF CtDNA resistance often suggests that the meningeal metastasis resistance mechanism is different from the primary tumor. This may be due to the following reasons: (I) It is difficult for TKI to pass the blood-brain barrier to form a certain concentration in CSF; (II) there is heterogeneity in different parts of drug resistance genes, including T790M (14-16); or (III) the existence of another resistance mechanism: secondary resistance mutations, activation of bypass signaling tracks, dysregulation of downstream effector proteins, and phenotypic transformation. For our patient, a clear new meningeal metastasis occurred during the oral administration of Tagrisso, which prompted us to ask whether he had resistance to other drugs. Consequently, NGS evaluated CSF CtDNA to understand the resistance mechanism of this patient. It revealed the presence of the EGFR 21 L858R mutation, combined with the T790M mutation, and that there were no other resistance mechanisms. The next question we will address is how to choose treatments under the premise of poor PS score and new meningeal metastasis during oral TKI treatment when the mechanism of drug resistance has not changed.
Treatment analysis of patients with meningeal metastasis
Treatment methods for CNS metastasis are currently limited, especially for patients with meningeal metastasis. There is no standardized diagnostic or treatment standard. Using EGFRTKI, chemotherapy, whole brain radiotherapy WBRT, intrathecal chemotherapy, and brain surgery has been reported (2,17-19).
The permeability of the blood-brain barrier (BBB) is influenced by many factors. Ballard et al. simulated different dose groups from 80 to 240 mg for brain metastases, and found that at least 80 mg of Tagrisso can reach the therapeutic dose, while 160 mg may be more effective (20). The clinical data of patients with meningeal metastasis treated with EGFR TKIs are very limited. Studies have reported that in a few cases, high-dose pulsed Erlotinib has achieved certain results in the treatment of meningeal metastasis (21). The few clinical and preclinical studies suggested that high-dose shock therapy may be a possible choice.
For this patient, we mainly considered the following two factors when formulating the next treatment strategy for this patient: (I) the results of NGS of CSF still showed EGFR 21 L858R mutation combined with T790M mutation without other resistance pathways, and the patient had developed disease progression during the oral dose of 80 mg qd which indicates that drug resistance had occurred. According to the patient’s situation, we considered whether the new meningeal metastasis was related to an insufficient concentration of TKIs in the CSF. High-dose TKIs may reach the therapeutic dose in the CSF after the concentration is increased in the plasma. (II) The patient’s PS score was 3, which meant that he could not tolerate treatment measures including chemotherapy or surgery, while whole brain radiotherapy does not prolong OS in meningeal metastasis patients (3). Based on the above, considering the possible hematological toxicity and gastrointestinal reactions of traditional chemotherapy, the strategy of Tagrisso 160 mg may be more suitable for this patient. However, it was a lack of a large sample to support the effect and safety of the treatment program, and the economic expenditure would also be double in the same period. After communication, patients expressed acceptable economic cost-utility, but concern about the unpredictable toxic side effects after the increase of Tagrisso. Therefore, we explained to him that the maximum tolerated dose of Tagrriso was 240 mg qd, and no new fatal AE occurred in the high dose group (22). Besides, the ongoing BLOOM clinical trial design, using Teresa 160 mg qd vs. AZD3759 to treat meningeal metastasis, showed that the safety and tolerability of the treatment are within the controllable range. The patient finally expressed understanding and accepted the treatment. After increasing Tagrisso to 160 mg qd, the patient’s neurological symptoms disappeared. The chest lesions were maintained in SD, the patient’s PFS was extended by 7 months, and no intolerable toxicities or side effects occurred throughout the course.
Conclusions
Throughout the entire treatment, the patient expressed support and gratitude for our medical decisions. Initially, he felt nervous and scared. However, the effect of the first chemotherapy and TKI treatments made him feel very optimistic about the prognosis. After the patient experienced local progression, the palliative radiotherapy we selected significantly improved his clinical symptoms, and he showed high trust and good compliance with the clinician. The appearance of CNS-related symptoms increased the patient’s anxiety once more. However, the next effective Tagrisso treatment alleviated the psychological pressure felt by the patient. In the meantime, we also informed the patient of the limitations of his treatment. After experiencing CNS-related symptoms again, the patient opted for palliative care at home.
In summary, in patients with advanced NSCLC, especially those with EGFR mutations, who develop CNS-related symptoms, clinicians should be highly alert to the possibility of meningeal metastasis. CSF biopsy is an effective diagnostic method and effective way to understand the resistance mechanism of meningeal metastasis in patients with EGFR mutations. As for patients with EGFR-sensitive mutations, increasing the dose of targeted drugs presents an alternative treatment option.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-451/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-451/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional review board of Shanghai Chest Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ostrom QT, Wright CH, Barnholtz-Sloan JS, et al. Brain metastases: epidemiology. Handb Clin Neurol 2018;149:27-42. [Crossref] [PubMed]
- Liao BC, Lee JH, Lin CC, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol 2015;10:1754-61. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Omuro AMP, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 2005;103:2344-8. [Crossref] [PubMed]
- Zhao J, Chen M, Zhong W, et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer 2013;14:188-93. [Crossref] [PubMed]
- Chamberlain MC. Leptomeningeal metastases in the MRI era. Neurology 2011;76:200. [Crossref] [PubMed]
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg Neurol Int 2013;4:S265-88. [Crossref] [PubMed]
- Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol 2006;5:443-52. [Crossref] [PubMed]
- Jiang BY, Li YS, Guo WB, et al. Detection of driver and resistance mutations in leptomeningeal metastases of NSCLC by next-generation sequencing of cerebrospinal fluid circulating tumor cells. Clin Cancer Res 2017;23:5480-8. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med 2017;376:2109-21. [Crossref] [PubMed]
- Birkbak NJ, McGranahan N. Cancer genome evolutionary trajectories in metastasis. Cancer Cell 2020;37:8-19. [Crossref] [PubMed]
- Tang WF, Wu M, Bao H, et al. Timing and origins of local and distant metastases in lung cancer. J Thorac Oncol 2021;16:1136-48. [Crossref] [PubMed]
- De Mattos-Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015;6:8839. [Crossref] [PubMed]
- Hata A, Katakami N, Yoshioka H, et al. Spatiotemporal T790M heterogeneity in individual patients with EGFR mutant non-small-cell lung cancer after acquired resistance to EGFR-TKI. J Thorac Oncol 2015;10:1553-9. [Crossref] [PubMed]
- Togashi Y, Inui K, Mishima M, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol 2010;5:950-5. [Crossref] [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Lee SJ, Lee J, Nam D, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol 2013;8:185-91. [Crossref] [PubMed]
- Riess JW, Nagpal S, Iv M, et al. Prolonged survival of patients with non-small-cell lung cancer with leptomeningeal carcinomatosis in the modern treatment era. Clin Lung Cancer 2014;15:202-6. [Crossref] [PubMed]
- Morris PG, Omuro AM, Reiner AS, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol 2012;7:382-5. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- How J, Mann J, Laczniak AN, et al. Pulsatile erlotinib in EGFR-positive non-small-cell lung cancer patients with leptomeningeal and brain metastases: review of the literature. Clin Lung Cancer 2017;18:354-63. [Crossref] [PubMed]
- Yang JCH, Kim SW, Kim DW, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: The BLOOM Study. J Clin Oncol 2020;38:538-47. [Crossref] [PubMed]




