Lung cancer treatment outcomes in recipients of lung transplant
Introduction
Lung transplant is utilized as a last resort and can be an effective treatment for patients with end stage lung disease. The incidence of lung cancer in lung transplant recipients is higher than that in general population (1-5). Contributing factors include significant smoking history, which is commonly noted in patients with lung cancer. Chronic immunosuppressed status after transplant is also thought to be a contributing risk factor (6). As the number of patients undergoing lung transplantation is increasing, more bronchogenic carcinomas are being detected after lung transplantation. There is a paucity of literature regarding treatment outcomes among lung transplant survivors who are diagnosed with lung cancer. As a result, there is a lack of consensus on the best treatment strategy for this rare population of patients.
We have conducted a retrospective review on a large cohort of lung transplant patients to identify the incidence and treatment outcomes after a lung cancer diagnosis among lung transplant recipients.
Methods
The Cleveland Clinic lung transplantation database was used to identify patients with lung cancer diagnosed at the time of or after lung transplant. Clinical information including demographics, underlying lung disease, type of transplant, immunosuppressive regimen, lung cancer pathologies, cancer-related treatment, response, and toxicities were retrospectively reviewed and collected. Survival time was calculated from the diagnosis of lung cancer to expiration or last date patient known to be alive. In patients who received chemotherapy for lung cancer, survival was also calculated from the initiation of chemotherapy to expiration or last known alive. The study was approved by the Institutional Review Board of Cleveland Clinic Foundation.
Results
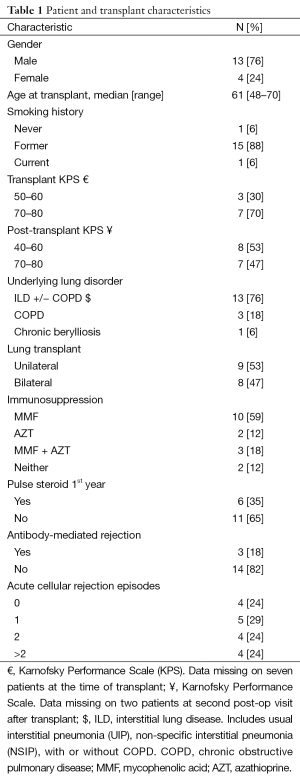
Between 2005 and 2013, 847 patients underwent a single or bilateral lung transplant at Cleveland Clinic Foundation. Of these patients, 17 (2%) were diagnosed with lung cancer subsequent to the transplant and were included in the study. Patient demographics and transplant characteristics are shown in Table 1. Most of the patients were male (76%) and former smokers (88%). The median age at lung transplant was 61 (range, 48–70) years. The majority of patients had lung transplantation for interstitial lung disease with or without chronic obstructive pulmonary disease (COPD) (76%). Nearly half of the patients underwent bilateral lung transplant (47%).
Full table
Tumor location and histology
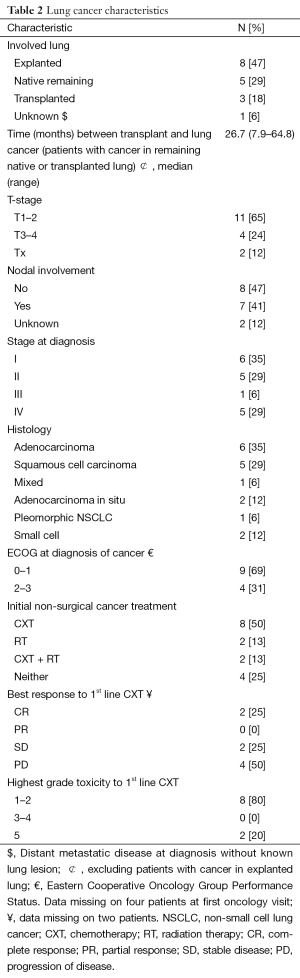
Eight cancers were detected in the explanted lung at the time of lung transplantation (47%), five were diagnosed in the remaining native lung (29%), and three cancers were later diagnosed in the transplanted (donor’s) lung (18%). One patient had lung cancer present as distant metastasis without an identified primary lesion (6%). Lung cancers detected in the native and transplanted lung were diagnosed at a median of 26.7 months (range, 7.9 months–5.4 years) after the transplant. The majority of patients (n=11) had early stage disease (stage I/II) (64%), one patient had locally advanced stage IIIA (6%) and five (29%) were stage IV disease at the time of diagnosis. Most patients with early stage disease were detected in the explanted lung at the time of transplant (n=8/11), few in the transplanted lung (n=2/11), and only one patient in the native lung after a single transplant.
Non-small cell lung cancer (NSCLC), was more common (n=15, 88%) than small cell lung cancer (SCLC) (n=2, 12%). NSCLC histology included squamous cell carcinoma (n=6), adenocarcinoma (n=6), adenocarcinoma in situ (n=2), and pleomorphic NSCLC (n=1). Lung cancer characteristics and treatment outcomes are summarized in Tables 2 and 3.
Full table

Full table
Treatment with a curative intent
Overall 12 out of 17 patients had potential curable disease (stage I–IIIA) and except for one patient, who did not receive adjuvant chemotherapy for stage II disease, all others were treated with stage-appropriate treatment. Among 12 patients with potential curable disease, eight patients had cancer in explanted lung and did not receive adjuvant therapy if diagnosed with stage I disease (5/8 explanted lung cancers were stage I). Two of these patients with stage I disease in explanted lung later developed relapsed disease in their transplanted lung (40%) and at that point, both patients were treated with chemotherapy. One patient also received additional palliative radiation.
Curative oncologic treatment beyond removal of explanted lung in stage I disease was offered based on stage and site of disease, and included lobectomy, radiation, sequential radiation followed by chemotherapy, and adjuvant chemotherapy. Lobectomy was performed in a patient with T2N1M0, stage II lung cancer in remaining native lung; however, this patient developed metastases 5 months later, and died 7 months after chemotherapy was initiated. Sequential radiation (50 Gy in 5 fractions) followed by chemotherapy was delivered to two patients with cancer in transplanted lung (one with stage I small cell lung cancer, and the other with stage II NSCLC disease). Adjuvant chemotherapy was administered in 2 out of 3 patients with stage II disease in explanted lung.
Chemotherapy
Chemotherapy was given in 10/17 (59%) all patients, primarily carboplatin-based doublets (n=8), paired with docetaxel, pemetrexed, or etoposide.
Upfront chemotherapy in the form of adjuvant treatment or combined with radiation was offered in 40% of all chemotherapy-treated patients (4/10) and the remaining 60% were treated with a palliative intent (6/10), half of whom were initially stage I or II at diagnosis. The remaining seven patients (41%) who were not treated with chemotherapy during their cancer treatment included four patients with early stage cancer in explanted lung (three with T1N0 stage I and one with T3N0 stage II), one patient with stage IIIA disease in remaining native lung treated with radiation alone, and two patients with stage IV metastatic disease.
Among patients with early stage disease (stage I–II, n=11), a total of seven patients received chemotherapy +/− radiation for either nodal involvement in the explanted lymph nodes (n=2), disease in the transplanted lung after bilateral transplant (n=2), or later, when metastases developed (n=3).
Among those patients who received chemotherapy with a palliative intent for metastatic disease (n=6), best radiographic response was progressive disease during treatment in three (50%) and not evaluable in two other patients (33%), however, one patient had a complete response 3 months after initiation of chemotherapy. Among patients with measurable disease who were given chemotherapy with curative intent (n=2), one had a complete response after sequential radiation followed by chemotherapy for SCLC, and the other had progression of disease as best response after receiving sequential radiation and chemotherapy for stage II squamous cell disease in transplanted lung.
Treatment outcome (Table 4)
Full table
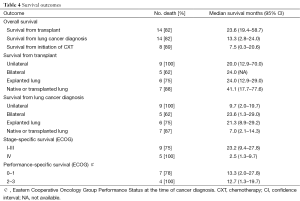
Median survival from the diagnosis of lung cancer was better in patients who had cancer in the explanted lung than in those who developed cancer at a later date in the native or transplanted lungs: 24 months [confidence interval (CI), 12.9–29 months] vs. 4.8 months (CI, 1.3–13.3 months). Unsurprisingly, median survival is also more favorable in stage I–IIIA (23.2 months; CI, 9.4–27.8 months) compared to stage IV disease (2.5 months; CI, 1.3–9.7 months).
Median survival of early stage patients (stage I–II) who received treatment was 14.3 months (range, 3.2–27.9 months). Two died of grade 5 neutropenia and sepsis at 3 and 13 months after the diagnosis of cancer. Also of note, two patients had a complete response to first-line chemotherapy. One was still alive 14.3 months after cancer diagnosis (when data was collected). The other patient unfortunately developed lung rejection at which point chemotherapy had to be stopped, with a survival time of 27.9 months after the diagnosis of lung cancer, 10 months after the initiation of chemotherapy. Early stage patients without treatment (n=4) had a median survival of 21 months (range, 8.9–29.2 months), two of whom died of causes unrelated to lung cancer (one due to lung rejection, and one due to sudden death of unknown cause).
Median survival of patients with stage IV metastatic disease at diagnosis was 2.7 months (range, 1.3–9.8 months) and those who received chemotherapy had slightly longer survivals (2.1–9.8 months) compared to those who did not receive chemotherapy (1.3 and 2.7 months). Of those patients who received chemotherapy, best response was documented as progression of disease in two patients (survival 4.7, and 9.8 months from diagnosis), and early death secondary to sepsis after first cycle of chemotherapy in third patient (survival 2.1 months). Due to small number of patients (n=5), no direct statistical comparison was performed among patients treated with chemotherapy vs. best supportive care only.
Patients who later developed metastatic disease, however, had a mixed response subsequent to chemotherapy. Three patients with early stage I–II who later developed metastasis were treated with chemotherapy. One patient with NSCLC had a complete response to therapy, but later discontinued continuous maintenance treatment due to transplant rejection. This patient lived to 10 months after initiation of chemotherapy (27.9 months since initial diagnosis of lung cancer). Another patient developed pancytopenia after two cycles of chemotherapy, did not have a formal response evaluation, and was switched to erlotinib. Unfortunately, this patient had progression of disease through erlotinib and lived for 5.5 months since starting systemic therapy (6.7 months since diagnosis of metastatic disease, 13.3 months from diagnosis of early stage lung cancer). The third patient had progression of disease as best response to chemotherapy with a poor survival since diagnosis of metastatic disease and initiation of chemotherapy (5.7 and 2.6 months, respectively; 19.4 months overall survival since diagnosis of cancer). The initial gap between diagnosis of metastatic disease and initiation of systemic treatment in this patient was due to confirmation and palliative radiation to soft tissue.
In general, patients who received chemotherapy had a median survival of 7.5 months (95% CI, 0.3–20.6 months) from the initiation of chemotherapy. Anemia (30%) and fatigue (30%) were the most common toxicities, while sepsis was most severe and the cause of death in 30% of chemotherapy-treated patients (n=3/10).
Discussion
The reported rate of developing lung cancer after lung transplantation is 1.0–4.1% (1,3,4,7), much higher than that of 0.2% in the general population (5). The incidence of lung cancer in our cohort was 2%, which is in line with prior studies. Review of available literature shows lung cancer to be more commonly detected after single-lung transplantation compared to bilateral lung transplant. The incidence of developing lung cancer in patients with single lung transplant is 6.9–9.8%, whereas that after bilateral lung transplant it is 0–1.8% (4,8). Most of the bronchogenic carcinomas were found in the native lung after single lung transplant (1,3,4,7-10). In contrast, in our study, only 5 out of 17 cases developed cancer in native lung after single transplant; 3 of whom were detected at an advanced stage with metastases to bone (n=3) and liver (n=2). In comparison, our study identified a significant number of cancers detected in explanted lungs (n=8), all associated with early stage disease with a better prognosis [median survival 21 months (range, 8.9–29.2 months)] compared to cancer in the native or transplanted lung [median survival 7.2 months (range, 2.1–14.3 months)]. The incidence of lung cancer detection in explanted lungs is 0.9% in our study population, which is again, in concordance with previous studies reporting an incidence of 0.8–2.0% of lung cancer found in explanted lungs (1,11).
Literature regarding the treatment of lung cancer in lung transplant recipients is scant. Treatment option for this group of patient is often limited given their immunosuppressive status and comorbidities. In our study, patients with advanced stage disease generally had poor survival regardless of chemotherapy. The longest surviving patient with stage IV upon diagnosis (survival 9.8 months from diagnosis) had small cell lung cancer and received chemotherapy with carboplatin/etoposide. The other four patients with metastatic disease at diagnosis had NSCLC with survival ranging between 1.3 to 4.7 months.
Patients who later developed metastatic disease, however, had a mixed response subsequent to chemotherapy. Three patients with stage I–II who later developed metastasis were treated with systemic therapy with best response measured as complete response in one, progression of disease in another, and not evaluable in the third case. The longest surviving patient was the one with complete response (10 months since initiation of chemotherapy) who eventually succumbed to transplant rejection rather than malignancy.
Overall, it appears that response to chemotherapy in the palliative setting is very low in this population, although occasional cases of complete response can be found. The more challenging aspect of treatment, however, remains the high toxicity rate with hematologic grade 4–5 events being common. Patients who were treated with chemotherapy numerically had a longer survival than those who were not treated, but it is difficult to control for the possible difference in comorbidities and performance in this retrospective review. In addition, it appears that patients who were initially diagnosed with early stage disease may have a better response to palliative chemotherapy when they develop metastasis compared to those with stage IV at diagnosis, however, risk of hematologic toxicities remain high in this population as well.
Patients with early stage disease detected in explanted lungs without nodal involvement had a reasonable survival without immediate additional treatment. Among five patients with stage I lung cancer discovered in explanted lung, two were alive at the time of data capture (9 and 19 months since diagnosis of cancer), and remaining patients had lived a median survival of 23.3 months (range, 19.4–27.9 months).
In addition, chemotherapy could be offered to those early stage patients with nodal involvement, those with disease in transplanted lung or remaining native lung after unilateral lung transplant, or patients with relapsed disease. Chemotherapy in this group results in higher rates of response and longer survival compared to those patients receiving therapy for stage IV disease. However, median survival in this group is numerically worse than those with stage I lung cancer in explanted lung, who did not receive chemotherapy. This difference to some extent reflects biologic and stage impact of nodal involvement in chemotherapy-treated group or comorbidities associated with cancer in remaining native or transplanted lung as compared to unexpected discovery of cancer in explanted lung without nodal involvement. It is also possible that some of the survival differences could be attributed to adverse events from chemotherapy, and particularly sepsis secondary to profound bone marrow suppression in this already immune-compromised population.
In summary, the possible survival advantages of chemotherapy must be weighed carefully against serious adverse events. Patients with metastatic disease had a numerically longer survival when treated with chemotherapy, and there have been cases of complete response in our cohort of chemotherapy-treated patients. But chemotherapy-associated toxicities can also be detrimental in this group of patients, as 30% of chemotherapy-treated patients died of grade 5 sepsis. Therefore modified regimens with careful dose reduction along with bacterial and viral prophylaxis should be considered when treating this group of patients in order to decrease the risk of severe toxicities.
Conclusions
Lung cancer detected at the time of or after lung transplantation carries various clinical courses. Patients with metastasis have poor survival and significant toxicities from chemotherapy, but variability in outcome still remains. Early stage patients and those with lung cancer in explanted lungs should be considered for treatment with a curative intent, but modified and dose-reduced regimens along with prophylactic antibiotics are highly encouraged to decrease the chance of severe adverse events, including profound neutropenia and sepsis.
Acknowledgements
None.
Footnote
Conflicts of Interest: NA Pennell—Boehringer Ingelheim: consultant; Genentech: consultant; N Hashemi-Sadraei—Gennetech: speaker, advisory board. The other authors have no conflicts of interest to declare.
References
- Arcasoy SM, Hersh C, Christie JD, et al. Bronchogenic carcinoma complicating lung transplantation. J Heart Lung Transplant 2001;20:1044-53. [PubMed]
- Choi YH, Leung AN, Miro S, et al. Primary bronchogenic carcinoma after heart or lung transplantation: radiologic and clinical findings. J Thorac Imaging 2000;15:36-40. [PubMed]
- Collins J, Kazerooni EA, Lacomis J, et al. Bronchogenic carcinoma after lung transplantation: frequency, clinical characteristics, and imaging findings. Radiology 2002;224:131-8. [PubMed]
- Dickson RP, Davis RD, Rea JB, et al. High frequency of bronchogenic carcinoma after single-lung transplantation. J Heart Lung Transplant 2006;25:1297-301. [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Penn I. Cancer in immunosuppressed patients. Transplant Proc 1984;16:492-4. [PubMed]
- Raviv Y, Shitrit D, Amital A, et al. Lung cancer in lung transplant recipients: experience of a tertiary hospital and literature review. Lung Cancer 2011;74:280-3. [PubMed]
- Yserbyt J, Verleden GM, Dupont LJ, et al. Bronchial carcinoma after lung transplantation: a single-center experience. J Heart Lung Transplant 2012;31:585-90. [PubMed]
- Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol 2008;3:1404-9. [PubMed]
- Anyanwu AC, Townsend ER, Banner NR, et al. Primary lung carcinoma after heart or lung transplantation: management and outcome. J Thorac Cardiovasc Surg 2002;124:1190-7. [PubMed]
- Abrahams NA, Meziane M, Ramalingam P, et al. Incidence of primary neoplasms in explanted lungs: long-term follow-up from 214 lung transplant patients. Transplant Proc 2004;36:2808-11. [PubMed]


