
Optimizing the clinical management of EGFR-mutant advanced non-small cell lung cancer: a literature review
Introduction
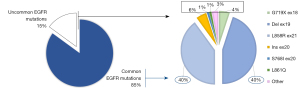
Over the past few decades, the identification of oncogenic drivers predicting clinical response to targeted therapies produced a radical shift from histological to molecular subtyping of lung adenocarcinoma, establishing a new paradigm of precision medicine. Tumor molecular profiling is now considered as a crucial step of the diagnostic and therapeutic management of advanced non-small cell lung cancer (NSCLC), allowing to personalize therapeutic strategies and ultimately improve patients’ survival (1). The epidermal growth factor receptor (EGFR) gene activating mutations represented the first molecular predictive biomarker discovered in lung cancer in 2004, underlying clinical responsiveness to the EGFR-tyrosine kinase inhibitors (TKIs) (2,3). Molecular alterations within the EGFR gene have been reported in about 40–60% and 12–15% of Asiatic and Caucasian adenocarcinoma patients, respectively, rarely occurring also in squamous cell carcinoma subtype (4). The most common targetable alterations include both exon 19 in frame deletion (Del19) and exon 21 L858R point mutation, accounting for about 85–90% of the overall EGFR mutations, predicting meaningful response to targeted agents. Conversely, uncommon alterations include a wide spectrum of mutations, deletions, as well as insertions, occurring among exons 18 to 21 of the EGFR gene (Figure 1), which are characterized by high heterogeneity in terms of response/resistance to the available EGFR-TKIs (4). Sometimes uncommon variants may occur at subclonal level, coexisting with either common or uncommon EGFR mutations, to define the “complex/compound” molecular subtypes. Co-occurring genomic alterations have been also reported in a significant subset of patients, highlighting the biological heterogeneity of the EGFR-mutant disease (5). Despite several steps forward in the treatment of EGFR-positive NSCLC, however there are still pending issues and upcoming challenges requiring adequate addressing. The increased detection of uncommon EGFR mutations following the advent of next-generation sequencing (NGS) in the real-word practice, along with the clinical development of novel selective inhibitors, highlighted the issue of adequate selection of the best EGFR-TKI to the right patient. The advent of osimertinib in first-line has dramatically changed the spectrum of both innate and acquired resistance mechanisms related to the EGFR-TKI therapy, accelerating the clinical investigation of novel treatment strategies as well as upfront combinations. The recent approval of potent, selective EGFR exon-20 insertions inhibitors questioned the diagnostic accuracy of old-standard genomic sequencing technologies, pushing the implementations of NGS-based molecular profiling in the real word practice scenario.
In this review we summarize the most recent findings regarding diagnostic testing and therapeutic strategies of EGFR-mutant NSCLC, in order to provide evidence-based answers to the aforementioned challenges, aiming to optimize the clinical management of metastatic patients harboring molecular alterations within the EGFR gene. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-1/rc).
Methods
Literature search was conducted using MEDLINE/PubMed, EMBASE, Scopus and Cochrane Library databases, up to December 2021. The following keywords were used as literature search terms: lung cancer, non-small cell lung cancer, epidermal growth factor receptor, targeted therapy, resistance, next generation sequencing. Relevant studies in English language published between 2004 and 2021 were selected. The literature retrieval was supplemented by manual searches of abstracts meeting proceedings, including the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) congress, as well as the World Conference on Lung Cancer (WCLC). Two authors (FP and PB) independently selected studies and disagreements were discussed and solved with a third author (GVS) (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 12th December, 2021 |
| Databases and other sources searched | MEDLINE/PubMed, EMBASE and Cochrane Library Databases. ASCO, ESMO, WCLC abstracts meeting proceedings |
| Search terms used (including MeSH and free text search terms and filters) | Lung cancer, non-small cell lung cancer, epidermal growth factor receptor, targeted therapy, resistance, next generation sequencing |
| Timeframe | 1st January 2004 to 12th December 2021 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | Relevant studies in English language were selected |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Two authors independently selected studies and disagreements were discussed and solved with a third author |
| Any additional considerations, if applicable | Not available |
Selecting the best EGFR-TKI to the right patient
TKIs have shown their superiority over platinum-based chemotherapy in terms of progression-free survival (PFS) and overall response rate (ORR) in several phase 3 clinical trials conducted in patients with EGFR-mutation positive advanced NSCLC (6). Moreover, TKIs are better tolerated and improve patients’ quality of life as compared to cytotoxic chemotherapy (6). The first generation of drugs comprises reversible inhibitors, such as gefitinib, erlotinib, and icotinib. Although characterized by some differences in terms of toxicity, these drugs have been considered the standard of care of EGFR+ advanced NSCLC for a long time. Afatinib is a second-generation, irreversible EGFR-TKI, which demonstrated longer time-to-treatment failure (TTF) and PFS as compared to gefitinib in a randomized phase IIB clinical trial (7). However, the study did not reach the co-primary end-point of OS, since afatinib failed to demonstrate a significant survival advantage in the overall study population [hazard ratio (HR) =0.86, 95% confidence interval (CI): 0.66–1.12]. Dacomitinib, another second-generation, irreversible EGFR-TKI, showed its superiority over gefitinib in a phase 3, randomized trial, in terms of both OS (HR =0.75, 95% CI: 0.59–0.94, P=0.0155) and PFS (HR =0.59, 95% CI: 0.47–0.74, P<0.0001), although with an increased toxicity (8,9). Finally, the randomized phase 3 FLAURA trial compared first-generation TKIs (gefitinib or erlotinib) with the third-generation inhibitor, osimertinib in patients with advanced NSCLC harbouring EGFR exon 19 deletions or exon 21 L858R mutation (i.e., “common” mutations) (10). Osimertinib is a potent, irreversible EGFR-TKI, highly selective for EGFR activating mutations as well as T790M resistance mutation. Moreover, unlike first-generation drugs, osimertinib is characterized by great ability to penetrate the blood-brain barrier (11). The double-blind FLAURA trial reached its primary end-point, as osimertinib significantly increased PFS as compared to first-generation TKIs (HR 0.46, 95% CI: 0.37–0.57; P<0.001). However, the experimental treatment did not increase ORR nor disease control rate (DCR). An update OS analysis, with a median follow-up of 39 months and 58% data maturity, showed a median of survival of 38.6 months for osimertinib and 31.8 months for the control arm (HR 0.80, 95% CI: 0.64–1.00; P=0.046) (12). Thanks to all these positive data, clinicians are now spoiled for choice. Indeed, treatment decisions should be based on drugs efficacy, expected toxicity profile, patients and disease characteristics as well as molecular data. When dealing with “common” EGFR mutations, the strongest data are coming from the phase 3 head-to-head FLAURA trial. Indeed, osimertinib significantly increase PFS with fewer adverse events (AEs) (grade 3: 34% vs. 45%) (10), although patients’ reported outcomes did not differ between the two treatment arms (13). Moreover, the high blood-brain barrier penetration of osimertinib, witnessed by the superior brain PFS (HR 0.48; 95% CI: 0.26–0.86; P=0.014), intracranial response rate (66% vs. 43%, odds ratio 2.5, 95% CI: 1.2–5.2; P=0.011) as well as the reduced de novo central nervous system (CNS) progression rate (6% vs. 15%), make this drug the best treatment option for patients with CNS disease (14). While these compelling data apply to patients with EGFR exon 19 deletions and exon 21 L858R point mutations, evidences for less common mutation subtypes are more scarce. Since it is clear that patients harbouring EGFR exon 20 insertions should not be candidated to the current available EGFR-TKIs, and deserve, as of today, upfront platinum-chemotherapy, novel selective inhibitors, such as mobocertinib and amivantamab, have recently demonstrated promising activity in phase I-II clinical studies (15,16), and are rapidly coming in the clinical setting. As regards patients with other, non-exon 20 insertions, EGFR uncommon mutations, the selection of first-line treatment should be accurately evaluated based on scientific evidence and patients’ characteristics. The largest clinical available datasets refer to afatinib, and suggest that this drug is somehow active against exon 18 G719X and E709X mutations, exon 19 insertions and missense mutations, exon 20 S786I and p.768I as well as exon 21 L861Q (17,18). More recently, small studies on osimertinib have been also reported, suggesting the role of this agent in most subgroups, with exception of exon 20 S786I and p.768I mutations (19,20). Moreover, in rare cases of de novo exon20 T790M mutations, osimertinib should be regarded as the treatment of choice. These data underline, once again, the need for a deep description of each specific EGFR molecular alteration to support clinicians in their treatment choices. As extended genomic profiling is becoming more common, the description of both compound mutations (i.e., concomitant EGFR mutations) and co-mutations (i.e., other genes mutations) are expected to increase in daily practice. While, at this point, we do not have strong data to support the use of one EGFR-TKI over another in patients harboring complex genotypes, ongoing studies will hopefully inform treatment choices in the next future. Indeed, although data about the negative predictive and prognostic role of TP53 mutations are conflicting, results of ongoing studies (NCT04695925) aiming at treatment intensification in this subgroup of patients are eagerly awaited (5).
Overcoming acquired resistance to osimertinib
The pre-existence of resistant clones and even more the onset of adaptive molecular aberrations influence the efficacy as well as the response duration to EGFR-TKI therapy. Over the past few years, we have witnessed radical changes in the biological landscape of EGFR-TKI related resistance, mostly due to the advent of osimertinib in first-line (21). A thorough understanding of both tumor heterogeneity and molecular background could help to build effective therapeutic strategies aiming to prevent as well as overcoming innate and acquired resistance to EGFR-TKIs.
The upfront administration of third generation TKI produced a dramatic decrease of on-target alterations, reported in about 10–15% of cases now as compared to 50–60% under both first- and second-generation EGFR-TKIs (22,23). The most common on-target resistance mechanism occurring during third-generation TKI therapy is the C797S mutation within the exon 20 of the EGFR gene, detected in about 7% of patients developing disease progression to Osimertinib in the FLAURA trial (23), while no T790M cases were detected in the same patients cohort (23). In absence of a coexisting T790M mutation, C797S mutation may potentially retain sensitivity to first/second-generation EGFR inhibitors, including gefitinib, erlotinib and afatinib. Conversely T790M and C797 coexisting tumor retain sensitivity to first or second-generations TKI only when such mutations occur in trans (on different alleles) (24). Fourth-generation EGFR inhibitors are currently under investigation, aiming to overcome EGFR-dependent mechanics of resistance (25). Different molecules (EAI001, EAI045 JBJ-04-125-02, DDC4002) have demonstrated in vitro and in vivo activity alone or in combination with third-generation TKI, not reaching yet the advanced stages of clinical development.
The analysis of paired pre- and post-osimertinib tumor samples by Schoenfeld et al., demonstrated that off-target resistance is more frequently reported in the first line osimertinib cohort, suggesting to be a time-dependent mechanism resulting in less durable responses to the third-generation TKI (26).
Among the off-target molecular alterations, the amplification of MET gene was the most frequent bypass pathway conferring acquired resistance to osimertinib, reported in about 10–15% of patients included in the FLAURA study (23). On this basis, early-phase clinical trials evaluated the efficacy of dual EGFR-MET inhibition strategy in this treatment context, showing preliminary promising results. The phase Ib TATTON study (NCT02143466) investigated the addition of the MET-TKI savolitinib to osimertinib in patients with both EGFR-mutant and MET-positive NSCLC patients failing prior EGFR-TKI. In the cohort B1, including 69 patients progressing to prior third-generation EGFR-TKIs, ORR and median PFS were 30% and 5.4 months, respectively (27). The phase 2 platform ORCHARD study is currently exploring the efficacy of molecularly-driven personalized treatments for EGFR-mutant NSCLC patients progressing to first-line osimertinib. Preliminary data from the small cohort of 17 EGFR-mutant patients harboring MET amplification showed a promising activity (ORR 40%) for the osimertinib and savolitinib combination (28), which is currently being investigated in the phase II SAVANNAH trial (NCT03778229). An alternative dual EGFR/MET targeting is provided by the use of bispecific antibodies. Particularly amivantamab (JNJ-61186372), a fully human EGFR-MET bispecific antibody with immune cell-directing activity, is currently being investigated in combination with the third-generations EGFR-TKI, lazertinib, within the CHRYSALIS phase I study. Preliminary data from the osimertinib-resistant cohort, showed an ORR of 36% and 41% in the molecularly unselected, platinum-naïve (n=45) and resistant (n=29) population, respectively. Higher rate of activity has been observed among those patients harboring either EGFR/MET-based resistance (ORR 47%, 8/17), as well as MET overexpression by immunohistochemistry (IHC) analysis (ORR 90%, 9/10) (29,30). A phase Ib CHRYSALIS-2 study is currently evaluating the safety of lazertinib as monotherapy or in combination with amivantamab (and platinum-based chemotherapy in a single cohort) in this treatment setting (NCT04077463). Although there is still lack of consensus regarding the definition of MET-positive disease as well as the optimal detection method, recent data coming from such studies suggested gene amplification as the more reliable biomarker to select best candidate for this treatment strategy.
HER3 is frequently overexpressed in the vast majority of lung cancer cells and is likely involved in the development of EGFR-TKI resistance occurrence. In a phase I trial including 57, heavily pretreated, TKI-resistant patients, a HER3 Directed Antibody Drug Conjugate (ADC), patritumab deruxtecan (U3-1402) showed promising activity, with an ORR of 39% and median PFS of 8.2 months in the overall unselected population (31). Clinical responses were observed across the spectrum of baseline HER3 protein expression as well as the different TKI resistance mechanisms, including EGFR C797S mutation, MET amplification, HER2 mutation, suggesting a potential efficacy regardless of tumor molecular landscape. A phase 2 prospective study is investigating single-agent patritumab deruxtecan after failure of EGFR TKIs and platinum-based chemotherapy therapy (NCT03260491). Other bypass pathways, included HER-2 amplification as well as molecular aberrations in the RAS-MAPK signaling pathway, reported in about 2% and 12–15% of NSCLC patients receiving upfront Osimertinib (23). Particularly, NRAS mutations were found in 1% of plasma samples from patients who received first-line osimertinib within the FLAURA study, while multiple KRAS mutations, including KRAS p.G12S and p.G12D have been reported in 3% of cases (23). Considering the RAS-MAPK signaling pathway, rare concomitant acquired BRAF V600E mutations and MET amplification have been described as a mechanism of resistance to first-line Osimertinib (32). Loss of PTEN seems to be a mechanism of primary resistance to EGFR TKIs, but has been also reported in cases of acquired resistance (33,34). Gene fusions involving driver oncogenes, such as ALK, RET, BRAF (23,35,36), as well as molecular alterations among cell cycle-related genes, such as amplification or mutations in cyclin D1, D2 and E1 genes, cyclin-dependent kinase (CDK) 4/6 and CDK inhibitor 2A genes, have been also detected in a significant subgroup of patients who progressed to first-line Osimertinib (23), and seem to be associated with negative outcomes and reduced EGFR-TKI activity (37), providing a rationale for combination therapies to overcome EGFR-TKIs resistance. Several other combinations with different drugs targeting different pathways (like CDK4/6 inhibitor, glutaminase inhibitor, JAK inhibitor, Bcl-2 inhibitor, aurora A kinase inhibitor, PARP inhibitor, AXL inhibitor, mTOR inhibitor) are currently under investigation in phase I/II clinical trial.
Tissue specimens’ analyses under third-generation EGFR-TKI therapy disclosed histological transformation to small cell lung cancer (SCLC), as off-target resistance mechanism arising in a substantially higher proportion of cases than previously reported with first- or second-generation inhibitors (15% vs. 3–9%) (26). SCLC transition in EGFR-mutant patients dramatically affects survival outcomes, leading to rapid disease progression and transient response to SCLC-directed chemotherapies (38). However, a comprehensive insight into the biological mechanisms underlying cancer cells’ phenotype switching is still wanting. The intratumor heterogeneity, in terms of morphological, genetic, and epigenetic background remains a crucial issue to be adequately addressed in this research context. Offin et al. demonstrated that patients harbouring co-occurring EGFR/RB1/TP53 alterations (5% of all EGFR-mutant lung cancers) are uniquely at risk for SCLC transformation during their disease course (35). Although not all EGFR/RB1/TP53-mutant NSCLC patients will transform to SCLC during their disease course, both RB1 and TP53 loss-of-function mutations appear to be necessary, but not sufficient, for lineage plasticity. Taking together, these observations suggested that identifying such three-gene mutational signature (EGFR/RB1/TP53) at diagnosis could provide an opportunity for early intervention trials in those patients at higher risk of SCLC transformation. On this rationale, clinical trials have been initiated to test the combination of EGFR TKIs upfront with conventional SCLC therapy (NCT03567642).
Today, single-agent immunotherapy should be considered only once both targeted and chemotherapy options have been exhausted. Like in non-oncogene addicted NSCLC, modulation of the immune response through PD-1 inhibition may be enhanced by the potential immunogenic effects of both antiangiogenic agents and cytotoxic chemotherapy. In this regards, sub-group analysis of EGFR mutant patients progressing to prior EGFR-TKI, from the phase III trial IMPOWER-150, revealed significantly survival benefit with the addition of atezolizumab and bevacizumab to platinum-chemotherapy (39). Another phase II prospective, single arm study has recently shown great antitumor activity of pembrolizumab in combination with platinum-based chemotherapy in EGFR-mutant NSCLC patients failing prior EGFR-TKI, (ORR of 42%, PFS of 8.3 months and OS of 22.2 months) (40) but conclusive data are expected from ongoing prospective randomized studies (NCT03515837).
Developing effective and tolerable upfront combinations
Aiming to improve the efficacy of EGFR-TKI therapy, different upfront combination strategies have been recently explored, including cytotoxic chemotherapy, anti-angiogenic agents, immune-checkpoint inhibitors, as well as novel selective drugs targeting different signaling pathways.
Preclinical data showed that combining chemotherapy with EGFR-TKIs may have a synergistic effect, resulting in the reduction of angiogenesis, along with the up-regulation of downstream EGFR signaling pathways, promoting the apoptosis of EGFR TKI-resistant cells (41,42).
The phase II NEJ005/TCOG0902 was the first randomized trial to investigate the efficacy of concurrent or sequential chemotherapy with gefitinib in 80 EGFR-positive NSCLC (43), showing a significantly longer OS, better PFS and similar ORR with the concurrent regimen compared to the sequential one (44). Han et al. evaluated platinum-based chemotherapy plus gefitinib versus either chemotherapy or gefitinib alone in 121 EGFR-positive NSCLC patients (45). The combination strategy provided longer PFS and OS compared to TKI alone. Noronha et al. evaluated platinum-chemotherapy plus gefitinib versus gefitinib alone in 350 untreated EGFR-positive NSCLC patients (46), revealing a clinical benefit in favour of the combination arm in terms of ORR and OS, with doubled PFS comparable to that observed within the FLAURA trial. The randomized phase III NEJ009 study evaluated gefitinib plus carboplatin-pemetrexed versus gefitinib alone (47), showing an improved RR, PFS and OS in favour of the experimental arm (Table 2). All the above mentioned studies showed that the addition of chemotherapy to the first-generation EGFR-TKI may be an effective strategy, increasing clinical responses and survival outcomes as compared to the EGFR-TKI alone. However the survival benefit associated to such combinations is similar to that obtained with single agent Osimertinib within the FLAURA trial. Therefore, considering the lack of CNS activity and the worse tolerability profile, characterized by an increased rate of severe AEs, especially the haematological toxicities, the use of such combinations is currently limited to specific countries where the clinical access to the third generation TKI is still denied. Limited data regarding the combination of osimertinib plus chemotherapy are currently available. Tanaka et al. tested osimertinib plus chemotherapy versus osimertinib alone in 62 T790M-positive NSCLC patients who failed prior EGFR-TKI (48). The study showed that the addition of chemotherapy to osimertinib was generally well tolerated without producing a significant impact on patients’ survival. A retrospective analysis of 18 EGFR-positive advanced NSCLC patients treated with the combination of osimertinib plus different chemotherapy regimens, confirmed a tolerable profile, showing a median duration of treatment comparable to that observed with osimertinib alone within the AURA3 trial (49). Preliminary safety data from the first 30 patients enrolled within the FLAURA2 study did not reveal new toxicity signals, supporting further investigation of osimertinib plus chemotherapy combinations in the upfront setting. Several studies are currently ongoing (Table 3) and the results will clarify soon the potential role of this treatment regimens at least in particular subgroups of EGFR-mutant NSCLC patients.
Table 2
| Author | Phase | Treatment arms | Patient (n) | ORR (%) | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|
| Sugawara et al., 2015 | II | Concurrent or sequential alternating regimen with gefitinib and platinum-based chemotherapy | 80 | 87.8 vs. 84.6 | 18.3 vs. 15.3; HR 0.71 (0.42–1.20; P=0.20) | 41.9 vs. 30.7; HR 0.51 (0.26–0.99; P=0.042) |
| Oizumi et al., 2018 | II | Concurrent or sequential alternating regimen with gefitinib and platinum-based chemotherapy | 80 | 90.2 vs. 82.1 | 17.5 vs. 15.3; HR 0.68 (0.42–1.12; P=0.130) | 41.9 vs. 30.7; HR 0.58 (0.34–0.97; P=0.036) |
| Han et al., 2017 | III | Platinum-based chemotherapy + gefitinib vs. gefitinib vs. platinum-based chemotherapy | 121 | 82.5 vs. 65.9 vs. 32.5 | 15.7 vs. 11.9 vs. 5.7; HR 0.48 (0.29–0.78; P=0.003); HR 0.16 (0.09–0.29; P<0.001) | 32.6 vs. 24.3 vs. 25.8; HR 0.46 (0.24–0.87; P=0.016); HR 0.36 (0.20–0.67; P=0.001) |
| Noronha et al., 2020 | III | Platinum-based chemotherapy + gefitinib vs. gefitinib | 350 | 75 vs. 63 | 16 vs. 8; HR 0.51 (0.39–0.66; P<0.001) | NR vs. 17; HR 0.45 (0.31–0.65; P<0.001) |
| Hosomi et al., 2020 | III | Platinum-based chemotherapy + gefitinib vs. gefitinib | 345 | 84 vs. 67 | 20.9 vs. 11.9; (HR 0.490; P<0.001) | 50.9 vs. 38.8; HR 0.722, P<0.01 |
EGFR, epidermal growth factor receptor; TKI, tyrosine-kinase inhibitor; NSCLC, non-small cell lung cancer; N, number; ORR, objective response rate; PFS, progression free survival; OS, overall survival; vs., versus; HR, hazard ratio.
Table 3
| ID (trial name) | Phase | Treatment arms | Primary endpoint | Status |
|---|---|---|---|---|
| First line | ||||
| NCT04035486 (FLAURA2) | III | Platinum-based chemotherapy + osimertinib vs. osimertinib | PFS | Recruiting |
| NCT03567642 | I | Osimertinib + platinum/etoposide | MDT | Recruiting |
| Second line | ||||
| NCT04765059 (COMPEL) | III | Platinum-based chemotherapy + osimertinib vs. platinum-based chemotherapy + placebo | PFS | Recruiting |
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression free survival; vs., versus; MDT, maximum dose tolerate.
Preclinical studies found that up-regulated EGFR signaling increased VEGF expression through hypoxia-independent mechanisms, while elevated VEGF contributed to the EGFR-TKIs resistance occurrence, providing biological rational for combination strategies. Four prospective randomized trials have already established the benefit of dual EGFR/VEGF pathways inhibition, showing a consistent efficacy in terms of median PFS (50-53) (Table 4). Both the NEJ026 and the RELAY trials demonstrated a significant survival benefit from the addition of bevacizumab and ramucirumab, respectively, to the first-generation EGFR-TKI erlotinib, leading to a median PFS that was comparable to that obtained with osimertinib within the FLAURA trial, and somewhat higher considering the subgroup of patients harboring the L858R mutation. However, three of these trials did not show any survival advantage while the final OS from the RELAY study is still pending. The randomized phase III BEVERLY trial has recently evaluated the addition of bevacizumab to erlotinib as first-line treatment for EGFR-mutated NSCLC (54), showing a significant PFS increase, without survival advantages as well as unexpected safety issues. In order to enhance the efficacy of osimertinib and delay the onset of acquired resistance, a phase I/II trial tested osimertinib plus bevacizumab as first-line in 49 EGFR-positive NSCLC patients, showing a median PFS of 19 months which is comparable to that obtained with osimertinib in the front-line setting (55). Kenmotsu et al. compared osimertinib plus bevacizumab versus osimertinib alone in 122 untreated EGFR-positive NSCLC patients, showing no differences in terms of median PFS. In the subgroup analysis, former-smoker (HR 0.481) and patients with Ex19del (HR 0.622) showed a trend toward a not significant increase of PFS in favour of the combination arm, as previously observed in other similar studies (56). Further prospective clinical trials are currently ongoing (Table 5) to confirm the hypothesis that the addition of antiangiogenic agents to osimertinib may improve first-line clinical outcomes at least in particular subsets of EGFR-mutant NSCLC.
Table 4
| Trial | Phase | Treatment arms | Patient (N) | ORR (%) | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|
| NEJ026 | III | Erlotinib + bevacizumab vs. erlotinib | 228 | 72 vs. 66 | 16.9 vs. 13.3; HR 0.605 (0.417–0.877; P=0.016) | 50.7 vs. 46.2; HR 1.00 (0.68–1.48) |
| ARTEMIS-CTONG1509 | III | Erlotinib + bevacizumab vs. erlotinib | 449 | 76 vs. 75 | 19.4 vs. 12.4; HR 0.59 (0.46–0.76; P<0.0001) | NR |
| RELAY | III | Erlotinib + ramucirumab vs. erlotinib | 449 | 76 vs. 75 | 19.4 vs. 12.4; HR 0.59 (0.46–0.76; P<0.0001) | NR |
| ACTIVE | III | Gefitinib + apatinib vs. gefitinib | 313 | 77.1 vs. 73.7 | 13.7 vs. 10.2; HR 0.71 (0.54–0.95; P=0.0189) | NR |
| BEVERLY | III | Erlotinib + bevacizumab vs. erlotinib | 160 | 81.3 vs. 52.5 | 15.4 vs. 9.7; HR 0.60 (0.42–0.85; P=0.0039) | 28.4 vs. 23.0; HR 0.70 (0.46–1.10, P=0.12) |
| NCT02803203 | I/II | Osimertinib + bevacizumab | 49 | 80 [67–91] | 19 (95% CI: 15–24) | 10.1 (6–NR, P=0.002) |
| WJOG9717L | II | Osimertinib + bevacizumab vs. osimertinib | 122 | 86 vs. 82 | 22.1 vs. 20.2; HR 0.86 (0.53–1.39; P=0.21) | NR vs. 22.1; HR 1.02 (0.43–2.44; P=0.96) |
EGFR, epidermal growth factor receptor; TKI, tyrosine-kinase inhibitor; NSCLC, non-small cell lung cancer; N, number; ORR, objective response rate; PFS, progression free survival; OS, overall survival; vs., versus; HR, hazard ratio.
Table 5
| ID (trial name) | Phase | Treatment arms | Primary endpoint | Status |
|---|---|---|---|---|
| First line | ||||
| NCT04988607 (FLAIR) | II | Osimertinib +/− bevacizumab | PFS | Not yet recruiting |
| NCT04425681 (OWBLM) | II | Osimertinib +/− bevacizumab | LM-PFS | Recruiting |
| NCT05104281 | II | Osimertinib +/− bevacizumab | PFS | Recruiting |
| NCT04974879 | II | Osimertinib +/− bevacizumab | PFS | Recruiting |
| NCT03909334 (RAMOSE) | II | Osimertinib +/− ramucirumab | PFS | Recruiting |
| NCT04181060 | III | Osimertinib ± bevacizumab | PFS | Recruiting |
| Second line | ||||
| NCT02789345 | I | Osimertinib + ramucirumab or necitumumab | DLT | Active, not recruiting |
| LY3009806-IIT-01 | Ib | Osimertinib + ramucirumab | DLT | Active |
| NCT03133546 (BOOSTER) | II | Osimertinib +/− bevacizumab | PFS | Active, not recruiting |
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression free survival; LM, leptomeningeal metastasis; DLT, dose limiting toxicity.
Preclinical models reported a potential interaction between EGFR activation and PD-L1 expression in the development of EGFR-TKI resistance. Therefore the combination of anti-PD-1/PD-L1 and EGFR TKIs might have synergistic effects in NSCLC therapy (57). The phase Ib TATTON study investigated the safety and tolerability of osimertinib plus durvalumab combination in EGFR-positive patients with disease progression on prior EGFR-TKI (58). In terms of toxicity, interstitial lung disease (ILD) was reported in 38% of patients, being higher than expected with either drug alone, including five patients (15%) with grade 3–4 AEs. Despite the efficacy results, the primary end-point of safety was not met and enrolment was early interrupted. The phase I/II study KEYNOTE-021 evaluated the combination of pembrolizumab plus either erlotinib or gefitinib in untreated EGFR-positive NSCLC patients (59). The study showed that pembrolizumab plus gefitinib was not feasible due to grade 3/4 liver toxicity in five out of seven patients (71.4%), leading to permanent treatment discontinuation in four patients, while pembrolizumab plus erlotinib produced similar AEs than erlotinib monotherapy. Another open-label multicenter phase I trial evaluated the combination of gefitinib and durvalumab in TKI-naive patients with EGFR-positive NSCLC (60), showing higher rate of toxicity without significant ORR/PFS improvement compared to gefitinib alone, as previously reported in similar populations. In contrast to these data, other early phase clinical trials of concurrent immunotherapy and TKIs have shown acceptable safety profiles. Rudin et al. evaluated the combination of erlotinib plus atezolizumab in EGFR TKI-naïve and pre-treated NSCLC patients (61). Grade 3 AEs occurred in 43% of patients and the most common were pyrexia and increased ALT. The combination demonstrated a manageable safety profile with promising efficacy results. The phase I CheckMate 012 trial evaluated nivolumab in combinations with different other targeted agents, including erlotinib for the EGFR-mutant advanced NSCLC cohort (62). Study results showed that the combination was effective and tolerable, with Grade 3 toxicities occurring in 24% of patients, and no grade 4/5 AEs (Table 6). In summary, this data suggested that the optimal sequencing of TKIs and immunotherapy in EGFR-mutant patients remains to be defined in order to minimize the risk of AEs and increase the clinical benefit.
Table 6
| Author | Phase | Treatment arms | Patients (N) | AEs (%) | ORR (%) | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|
| Oxnard et al., 2020 (TATTON) | Ib | Osimertinib + durvalumab | 23 | ILD 38 (13/34) | 67 (T790M+); 21 (T790M−) | NR | NR |
| Awad et al., 2021 | I/II | Erlotinib + pembrolizumab; Gefitinib + pembrolizumab | 12; 7 | Liver G3-4 70 (5/7) | 42; 14 | 19.5 (3.0–9.5); 1.4 (0.2–13.0) | NR (19.5–NR); 13.0 (0.2–NR) |
| Creelan et al., 2021 | I | Gefitinib + durvalumab | 56 | 20 (4/20) | 63.3 (43.9–80.1) | 10.1 (5.5–15.2) | NR |
| Gettinger et al., 2018 | I | Erlotinib + nivolumab | 21 | 24 (G3) | 15 [3–38] | 5.1 (2.3–12.1) | 18.7 (7.3–NR) |
| Rudin et al., 2018 | I | Erlotinib + atezolizumab | 28 | 43 (G3) | 75 [51–91] | 15.4 (8.4–NR) | 32.7 (32.7–NR) |
EGFR, epidermal growth factor receptor; TKI, tyrosine-kinase inhibitor; NSCLC, non-small cell lung cancer; N, number; AEs, adverse events; ORR, objective response rate; PFS, progression free survival; OS, overall survival; NR, not reached; G, grade.
Recently, the phase I CHRISALYS study showed impressive results from the upfront combination of lazertinib and amivantamab (29), yielding a 100% ORR in the small cohort of treatment naïve, EGFR mutant NSCLC patients. Based on this encouraging evidence, the randomized phase III MARIPOSA study (NCT04487080) is currently randomizing EGFR-positive advanced NSCLC patients to first-line lazertinib plus amivantamab versus either osimertinib or lazertinib alone, aiming to further increase the survival plateau set by Osimertinib in this patients’ subgroup. Other innovative combinations with EGFR TKIs and different molecules as poly (ADP-ribose) polymerase (PARP), Aurora kinase or CDK4–6 inhibitors are currently under investigation and the results are eagerly awaited.
Implementing next generation sequencing in the routine molecular testing
Molecular testing for EGFR mutations is a crucial step of the clinical management of lung cancer patients. Until 2015, Sanger sequencing and/or pyrosequencing were the most widespread detection methods used to assess the EGFR mutational status in advanced NSCLC (63). Sanger sequencing was considered as the gold standard approach for a long time, since enabling the evaluation of whole genes sequences as well as the identification of unknown mutations, including the analysis of small DNA fragments sequences (64). Major limitations include laboratory intensiveness, high costs, and low sensitivity, requiring almost 40–50% of tumour cells within the tested sample (64). Therefore several laboratories performed a technological shift to Pyrosequencing, characterized by an inferior limit of detection (5% vs. 20%) of mutant alleles, thus allowing the identification of individual bases or short stretches of nucleic acid sequences at predetermined positions. Specifically, the commercially available PS kits properly identifies the most common EGFR exons 18–21 mutations, while are not able to adequately cover all EGFR uncommon alterations (65). Quantitative real-time polymerase chain reaction (qRT-PCR) was largely adopted to detect either “common” or “uncommon” EGFR mutations in the real-world practice scenario (4). However recent work revealed that current commercially available PCR kits could miss around 50% of EGFR exon 20 insertion variants as compared to NGS-based molecular profiling (66). Despite its high specificity, an important limitation of RT-PCR consists of detecting only known and well characterized molecular alterations, similarly to other targeted-based approaches. Conversely NGS represents a highly sensitive and specific technology for the molecular assessment of less frequent mutations, allowing the simultaneous detection of several hotspot genes from different patients’ samples (67). Differently from targeted-based approaches, NGS is able to identify either known or unknown mutations within the gene panel reference range, offering higher diagnostic accuracy, faster turnaround time for low sample volumes, and lower costs (68). To date, several NGS panels are commercially available enabling the simultaneous analysis of a plethora of clinically relevant hotspots in target genes, including EGFR (69). Based on this evidence, ESMO has recently recommended NGS as new standard approach to routinely profile newly diagnosed advanced NSCLC patients with non-squamous histology (70), in order to ensure adequate detection of oncogenic drivers and subsequent assignment to matched targeted treatment. However a significant fraction of NSCLC patients across different countries do not undergo yet NGS-based molecular profiling, because of regulatory, economic, cultural, and logistics barriers which limit patients’ access to NGS-platforms (71,72). Particularly in Italy, only one third of molecular pathology laboratories declared to routinely use NGS for the molecular analysis of advanced NSCLC patients, while RT-PCR still represents the most adopted technique for EGFR mutation testing in clinical practice (4).
Since a long time the clinical assessment of EGFR mutations by circulating tumor (ct)DNA analysis has been considered as a reliable alternative to tissue genotyping for advanced NSCLC patients who cannot undergo tumor biopsy. Recently a series of diagnostic accuracy clinical studies have definitively demonstrated a high concordance between tissue and ctDNA NGS-based molecular profiling, leading to the subsequent approval of the first ctDNA NGS diagnostic assay for the molecular profiling of advanced NSCLC patients in the United States. The ctDNA NGS analysis has shown to accurately detect molecular mechanisms of acquired resistance under Osimertinib therapy, allowing longitudinal tracking of EGFR somatic alterations in EGFR-mutant NSCLC patients included in the FLAURA trial (23,73). The phase II ELIOS study (NCT03239340) is prospectively investigating the concordance between tumour tissue and ctDNA-based NGS analysis for the detection of resistance mechanisms to upfront osimertinib in EGFR-positive NSCLC patients, and the results are eagerly awaited. In the meantime, the International Association for the Study of Lung Cancer (IASLC) has recently proposed a novel diagnostic algorithm integrating both tissue and liquid biopsy for the molecular profiling of advanced NSCLC (74).
Conclusions
EGFR mutations represent an established therapeutic target in NSCLC, with different generations EGFR-TKIs already approved for the clinical treatment of patients harboring common mutations. Conversely the best therapeutic choices for patients harboring less common EGFR molecular alterations are still debated, while novel selective and effective exon 20 insertions inhibitors are coming soon in the clinical setting. With the recent introduction of Osimertinib in first-line, clinical challenges for thoracic oncologist consist of identifying therapeutic strategies to overcome innate and acquired resistance to third generation TKI. Among the different drugs/combinations under clinical investigation, the dual inhibition of EGFR/MET pathways is emerging as an effective strategy, especially for patients harboring MET amplification, while preliminary data regarding HER3 therapeutic targeting, showed a wide spectrum of activity across different resistance mechanisms, emerging as a potential option for patients who do not harbor MET amplification or may not undergo tumor re-biopsy, which remains currently recommended when clinically feasible. Looking at upfront combinations, available data regarding both chemotherapy and antiangiogenic associations with first-generation EGFR-TKI showed a clinical activity comparable to that observed with Osimertinib monotherapy within the randomized FLAURA trial. Therefore, considering the lack of CNS activity and the worse tolerability profile, characterized by an increased rate of severe AEs, the use of such combinations should be currently limited to specific countries where clinical access to the third generation TKI is still denied. A series of ongoing studies are investigating Osimertinib combinations with both anti-angiogenics and chemotherapeutic agents, and even if preliminary data emerging from second-line setting are quite discouraging, final results from first-line clinical trials are eagerly awaited. Among the most promising upfront combinations studies, the ongoing randomized MARIPOSA trial will inform us whether the combination of amivantamab and lazertinib will be able to further increase the survival plateau set by Osimertinib in the treatment of EGFR-mutant disease.
The increasing prevalence of uncommon alterations in the real word scenario and the upcoming advent of new targeted options against the exon20 insertions, highlight the necessity to implement a comprehensive NGS-based molecular profiling in advanced, non-squamous NSCLC patients, in order to adequately detect all EGFR molecular alterations and personalize therapeutic strategies. In this regards a recent work has proposed an alternative classification of EGFR mutations based on their structure and function, suggesting that functional-based subgroups might predict EGFR-TKI therapeutic response better than classical exon-based groups (75). In conclusion we are currently facing novel diagnostic challenges and therapeutic opportunities, which, if adequately addressed, will allow to further optimize the clinical management EGFR-mutant advanced NSCLC patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-1/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-1/coif). GVS serves as an unpaid editorial board member of Translational Lung Cancer Research from August 2021 to July 2023. FP declared consultant’s fee from: AstraZeneca, Janssen, Amgen, Thermo fisher Scientific. PB received honoraria as speaker’s bureau by Roche, Bristol-Myers Squibb, AstraZeneca, Takeda, Merck Sharp and Dohme; he declared expenses by Amgen and Daiichi Sankyo; he received institutional research funding from Roche and Pfizer. GVS received consultant’s fee from Roche, Pfizer, AstraZeneca, Eli Lilly, Takeda; he received institutional research funding from Eli Lilly, Tesaro, Bayer and Merck Sharp and Dohme. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020;383:640-9. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Malapelle U, Pilotto S, Passiglia F, et al. Dealing with NSCLC EGFR mutation testing and treatment: A comprehensive review with an Italian real-world perspective. Crit Rev Oncol Hematol 2021;160:103300. [Crossref] [PubMed]
- Bironzo P, Reale ML, Sperone T, et al. Clinical and Molecular Features of Epidermal Growth Factor Receptor (EGFR) Mutation Positive Non-Small-Cell Lung Cancer (NSCLC) Patients Treated with Tyrosine Kinase Inhibitors (TKIs): Predictive and Prognostic Role of Co-Mutations. Cancers (Basel) 2021;13:2425. [Crossref] [PubMed]
- Greenhalgh J, Boland A, Bates V, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev 2021;3:CD010383. [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Mok TS, Cheng Y, Zhou X, et al. Updated Overall Survival in a Randomized Study Comparing Dacomitinib with Gefitinib as First-Line Treatment in Patients with Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. Drugs 2021;81:257-66. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Xing P, Mu Y, Hao X, et al. Data from real world to evaluate the efficacy of osimertinib in non-small cell lung cancer patients with central nervous system metastasis. Clin Transl Oncol 2019;21:1424-31. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Leighl NB, Karaseva N, Nakagawa K, et al. Patient-reported outcomes from FLAURA: Osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer 2020;125:49-57. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Park K, John T, Kim SW, et al. Amivantamab (JNJ-61186372), an anti-EGFR-MET bispecific antibody, in patients with EGFR exon 20 insertion (exon20ins)-mutated nonsmall cell lung cancer (NSCLC). J Clin Oncol 2020;38:abstr 9512.
- Riely GJ, Neal JW, Camidge DR, et al. Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations from a Phase I/II Trial. Cancer Discov 2021;11:1688-99. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Yang JC, Schuler M, Popat S, et al. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J Thorac Oncol 2020;15:803-15. [Crossref] [PubMed]
- Cho JH, Lim SH, An HJ, et al. Osimertinib for Patients With Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J Clin Oncol 2020;38:488-95. [Crossref] [PubMed]
- Bar J, Kian W, Wolner M, et al. UNcommon EGFR mutations: International Case series on efficacy of Osimertinib in Real-life practice in first-liNe setting (UNICORN). Ann Oncol 2021;32:S961-S962. [Crossref]
- Yang Z, Yang N, Ou Q, et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2018;24:3097-107. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol 2018;29:VIII740.
- Wang Z, Yang JJ, Huang J, et al. Lung Adenocarcinoma Harboring EGFR T790M and In Trans C797S Responds to Combination Therapy of First- and Third-Generation EGFR TKIs and Shifts Allelic Configuration at Resistance. J Thorac Oncol 2017;12:1723-7. [Crossref] [PubMed]
- To C, Jang J, Chen T, et al. Single and Dual Targeting of Mutant EGFR with an Allosteric Inhibitor. Cancer Discov 2019;9:926-43. [Crossref] [PubMed]
- Schoenfeld AJ, Chan JM, Kubota D, et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin Cancer Res 2020;26:2654-63. [Crossref] [PubMed]
- Sequist LV, Han JY, Ahn MJ, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol 2020;21:373-86. [Crossref] [PubMed]
- Yu HA, Ambrose H, Baik C, et al. ORCHARD osimertinib + savolitinib interim analysis: A biomarker- directed phase II platform study in patients (pts) with advanced non-small cell lung cancer (NSCLC) whose disease has progressed on first-line (1L) osimertinib. Annal Oncol 2021;32:S949-S1039. [Crossref]
- Bauml J, Cho BC, Park K, et al. Amivantamab in combination with lazertinib in the treatment of osimertinib-relapsed, chemotherapy-naïve EGFR mutant non-small cell lung cancer and potential biomarkers for response. J Clin Oncol 2021;39:abstr 9006.
- Shu CA, Goto K, Ohe Y, et al. Amivantamab plus lazertinib in post-osimertinib, post-platinum chemotherapy EGFR-mutant non-small cell lung cancer (NSCLC): Preliminary results from CHRYSALIS-2. Annal Oncol 2021;32:S949-S1039. [Crossref]
- Janne PA, Baik C, Su WC, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated (EGFRm) non-small cell lung cancer (NSCLC). J Clin Oncol 2021;39:abstr 9007.
- Minari R, Bordi P, La Monica S, et al. Concurrent Acquired BRAF V600E Mutation and MET Amplification as Resistance Mechanism of First-Line Osimertinib Treatment in a Patient with EGFR-Mutated NSCLC. J Thorac Oncol 2018;13:e89-91. [Crossref] [PubMed]
- Lee J, Kim HS, Lee B, et al. Genomic landscape of acquired resistance to third-generation EGFR tyrosine kinase inhibitors in EGFR T790M-mutant non-small cell lung cancer. Cancer 2020;126:2704-12. [Crossref] [PubMed]
- Wang F, Diao XY, Zhang X, et al. Identification of genetic alterations associated with primary resistance to EGFR-TKIs in advanced non-small-cell lung cancer patients with EGFR sensitive mutations. Cancer Commun (Lond) 2019;39:7. [Crossref] [PubMed]
- Offin M, Somwar R, Rekhtman N, et al. Acquired ALK and RET Gene Fusions as Mechanisms of Resistance to Osimertinib in EGFR-Mutant Lung Cancers. JCO Precis Oncol 2018; [Crossref] [PubMed]
- Schrock AB, Zhu VW, Hsieh WS, et al. Receptor Tyrosine Kinase Fusions and BRAF Kinase Fusions are Rare but Actionable Resistance Mechanisms to EGFR Tyrosine Kinase Inhibitors. J Thorac Oncol 2018;13:1312-23. [Crossref] [PubMed]
- Blakely CM, Watkins TBK, Wu W, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet 2017;49:1693-704. [Crossref] [PubMed]
- Marcoux N, Gettinger SN, O'Kane G, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol 2019;37:278-85. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Gadgeel SM, Dziubek K, Nagasaka M. Pembrolizumab in combination with platinum-based chemotherapy in recurrent EGFR/ALK+ nonsmall cell lung cancer (NSCLC). J Thorac Oncol 2021;16:S863. [Crossref]
- Lee JG, Wu R. Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1α in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia 2015;17:190-200. [Crossref] [PubMed]
- Wu M, Yuan Y, Pan YY, et al. Combined gefitinib and pemetrexed overcome the acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Mol Med Rep 2014;10:931-8. [Crossref] [PubMed]
- Sugawara S, Oizumi S, Minato K, et al. Randomised phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations: NEJ005/TCOG0902. Ann Oncol 2015;26:888-94. [Crossref] [PubMed]
- Oizumi S, Sugawara S, Minato K, et al. Updated survival outcomes of NEJ005/TCOG0902: a randomised phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations. ESMO Open 2018;3:e000313. [Crossref] [PubMed]
- Han B, Jin B, Chu T, et al. Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: A randomized controlled trial. Int J Cancer 2017;141:1249-56. [Crossref] [PubMed]
- Noronha V, Patil VM, Joshi A, et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol 2020;38:124-36. [Crossref] [PubMed]
- Hosomi Y, Morita S, Sugawara S, et al. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J Clin Oncol 2020;38:115-23. [Crossref] [PubMed]
- Tanaka K, Asahina H, Kishimoto J, et al. Osimertinib versus osimertinib plus chemotherapy for non-small cell lung cancer with EGFR (T790M)-associated resistance to initial EGFR inhibitor treatment: An open-label, randomised phase 2 clinical trial. Eur J Cancer 2021;149:14-22. [Crossref] [PubMed]
- Piotrowska Z, Liu SV, Muzikansky A, et al. Safety of osimertinib plus chemotherapy in EGFR-mutant NSCLC. J Clin Oncol 2018;36:e21231. [Crossref]
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019;20:625-35. [Crossref] [PubMed]
- Zhou Q, Wu Y, Cheng Y, et al. CTONG1509: phase 3 study of bevacizumab with or without erlotinib in untreated Chinese patients with advanced EGFR-mutated NSCLC. Ann Oncol 2019;30:602-60. [Crossref]
- Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:1655-69. [Crossref] [PubMed]
- Zhao H, Yao W, Min X, et al. Apatinib Plus Gefitinib as First-Line Treatment in Advanced EGFR-Mutant NSCLC: The Phase III ACTIVE Study (CTONG1706). J Thorac Oncol 2021;16:1533-46. [Crossref] [PubMed]
- Piccirillo MC, Bonanno L, Garassino MCC, et al. Bevacizumab + erlotinib vs erlotinib alone as first-line treatment of pts with EGFR mutated advanced non squamous NSCLC: Final analysis of the multicenter, randomized, phase III BEVERLY trial. Ann Oncol 2021;32:949-1039. [Crossref]
- Yu HA, Schoenfeld AJ, Makhnin A, et al. Effect of Osimertinib and Bevacizumab on Progression-Free Survival for Patients With Metastatic EGFR-Mutant Lung Cancers: A Phase 1/2 Single-Group Open-Label Trial. JAMA Oncol 2020;6:1048-54. [Crossref] [PubMed]
- Kenmotsu H, Wakuda K, Mori K, et al. LBA44 - Primary results of a randomized phase II study of osimertinib plus bevacizumab versus osimertinib monotherapy for untreated patients with non-squamous non-small cell lung cancer harboring EGFR mutations: WJOG9717L study. Ann Oncol 2021;32:S1283-S1346. [Crossref]
- Tung JN, Lin PL, Wang YC, et al. PD-L1 confers resistance to EGFR mutation-independent tyrosine kinase inhibitors in non-small cell lung cancer via upregulation of YAP1 expression. Oncotarget 2017;9:4637-46. [Crossref] [PubMed]
- Ahn M-J, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11:S115. [Crossref]
- Yang JC, Gadgeel SM, Sequist LV, et al. Pembrolizumab in Combination With Erlotinib or Gefitinib as First-Line Therapy for Advanced NSCLC With Sensitizing EGFR Mutation. J Thorac Oncol 2019;14:553-9. [Crossref] [PubMed]
- Creelan BC, Yeh TC, Kim SW, et al. A Phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. Br J Cancer 2021;124:383-90. [Crossref] [PubMed]
- Rudin CA, Cervantes AA, Dowlati A, et al. Long-Term Safety and Clinical Activity Results from a Phase Ib Study of erlotinib Plus Atezolizumab in Advanced NSCLC. Ann Oncol 2018;S407.
- Gettinger S, Hellmann MD, Chow LQM, et al. Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC. J Thorac Oncol 2018;13:1363-72. [Crossref] [PubMed]
- Khoo C, Rogers TM, Fellowes A, et al. Molecular methods for somatic mutation testing in lung adenocarcinoma: EGFR and beyond. Transl Lung Cancer Res 2015;4:126-41. [PubMed]
- Heather JM, Chain B. The sequence of sequencers: The history of sequencing DNA. Genomics 2016;107:1-8. [Crossref] [PubMed]
- Righi L, Cuccurullo A, Vatrano S, et al. Detection and characterization of classical and “uncommon” exon 19 Epidermal Growth Factor Receptor mutations in lung cancer by pyrosequencing. BMC Cancer 2013;13:114. [Crossref] [PubMed]
- Bauml JM, Viteri S, Minchom A, et al. FP07.12 Underdiagnosis of EGFR Exon 20 Insertion Mutation Variants: Estimates from NGS-based Real-World Datasets. J Thorac Oncol 2021;16:S208-S209. [Crossref]
- Luh F, Yen Y. FDA guidance for next generation sequencing-based testing: balancing regulation and innovation in precision medicine. NPJ Genom Med 2018;3:28. [Crossref] [PubMed]
- Zhong Y, Xu F, Wu J, et al. Application of Next Generation Sequencing in Laboratory Medicine. Ann Lab Med 2021;41:25-43. [Crossref] [PubMed]
- Haynes BC, Blidner RA, Cardwell RD, et al. An Integrated Next-Generation Sequencing System for Analyzing DNA Mutations, Gene Fusions, and RNA Expression in Lung Cancer. Transl Oncol 2019;12:836-45. [Crossref] [PubMed]
- Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol 2020;31:1491-505. [Crossref] [PubMed]
- Kerr KM, Bibeau F, Thunnissen E, et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer 2021;154:161-75. [Crossref] [PubMed]
- Smeltzer MP, Wynes MW, Lantuejoul S, et al. The International Association for the Study of Lung Cancer Global Survey on Molecular Testing in Lung Cancer. J Thorac Oncol 2020;15:1434-48. [Crossref] [PubMed]
- Reungwetwattana T, Gray J, Markovets A, et al. Longitudinal circulating tumour DNA (ctDNA) monitoring for early detection of disease progression and resistance in advanced NSCLC in FLAURA. Ann Oncol 2019;30:ix199. [Crossref]
- Rolfo C, Mack P, Scagliotti GV, et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J Thorac Oncol 2021;16:1647-62. [Crossref] [PubMed]
- Robichaux JP, Le X, Vijayan RSK, et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature 2021;597:732-7. [Crossref] [PubMed]


