Immunotherapy in locally-advanced non-small cell lung cancer: releasing the brakes on consolidation?
Stage III locally-advanced non-small cell lung cancer (LA-NSCLC) represents approximately 30% of new NSCLC diagnoses per year. Treatment options include definitive chemoradiation (1) or surgery in combination with chemotherapy or chemoradiation (2). Despite aggressive treatment, 5-year overall survival is only 15–20%. Therefore, much work is needed to improve outcomes in this population.
Consolidation or maintenance chemotherapy given beyond what is administered concurrently with radiation therapy has not been shown to improve survival in multiple randomized trials. The use of two cycles of systemic dosing of consolidation carboplatin and paclitaxel after concurrent chemoradiation with the same agents using radiosensitizing dosing is generally recommended and commonly used in current cooperative group trials, despite lack of randomized data supporting this approach (3). Furthermore, the phase III PROCLAIM trial randomized patients to cisplatin and pemetrexed with concurrent radiotherapy followed by consolidation pemetrexed versus cisplatin and etoposide with concurrent radiotherapy followed by cytotoxic chemotherapy of choice in stage III non-squamous NSCLC and found no difference between the two arms (4); results of this trial reinforced that chemotherapy choice based on histology does not necessarily lead to better outcomes.
Given the limited success of traditional cytotoxic chemotherapies as maintenance therapy for LA-NSCLC, recent studies have investigated the role of novel agents as maintenance or consolidation. For example, there is an ongoing trial of nanoparticle albumin-bound paclitaxel (nab-P) after nab-P plus carboplatin in stage IIIB/IV squamous cell NSCLC (NCT02027428). Antiangiogenic agents such as bevacizumab (5) and thalidomide (3) have also been assessed but have been shown to be harmful in the consolidation setting. In addition, molecular therapy targeted against the epidermal growth factor receptor (EGFR) also has not been shown to improve outcomes after definitive chemoradiation. A phase III study did not show any benefit to maintenance gefitinib, an EGFR tyrosine kinase inhibitors (TKIs) (6). Likewise, Radiation Therapy Oncology Group (RTOG) 0617 was a 2×2 randomized study investigating radiation dose (60 vs. 74 Gy) and the use of consolidation cetuximab, a monoclonal antibody to EGFR. The 60 Gy arm was found to have better overall survival, and there was no survival benefit and more toxicities with consolidation cetuximab (7). However, these studies have been critiqued for their being tested in unselected populations. RTOG 1306 is underway to define the role of molecular therapy in patients with LA-NSCLC who have known EGFR mutations or ALK translocations, although targeted therapy is given as induction therapy and not as maintenance therapy or concurrently with radiation in this trial. Additional studies are looking at other pathways such as the inhibition of MEK downstream of the RAS oncogene pathway (NCT 01912625).
Immunotherapy has recently reshaped the standard of care in metastatic NSCLC. Immunotherapy can allow a patient’s immune system to recognize cancer cells as being foreign, which can trigger an immune response and resulting tumor cell death or growth inhibition (8). Broadly, immunotherapy can be categorized into checkpoint inhibitors, including antibodies to program death receptor-1 (PD-1), program death receptor ligand-1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). The PD-1 inhibitor, nivolumab, was first approved by the U.S. Food and Drug Administration (FDA) for second line treatment in squamous cell lung cancer after it was shown to have a significant survival advantage over docetaxel (9). Pembrolizumab, a PD-L1 inhibitor, also received FDA approval after it was shown in the KEYNOTE-001 study to have excellent antitumor efficacy, with an objective response rate of 19.4% and median duration of response 12.5 months; PD-L1 expression in at least 50% of tumor cells correlated with improved efficacy of the drug (10).
Although these drugs have predominantly been used for lung cancer as monotherapy, there is suggestion that immunotherapy may enhance the effects of radiotherapy, and vice-versa (11). As radiation causes tumor cell apoptosis and necrosis, release of tumor antigens may induce an anti-tumor immune reaction by upregulation of immunogenic cell surface markers (12-14). In addition, inflammation to normal tissue caused by radiotherapy may lead to either secretion of cytokines or an infiltration of tumor-specific T cells via pathways such as vascular normalization (15,16) or induction of pro-inflammatory chemokines (e.g., cxcl16) (17). Lastly, radiotherapy may also upregulate the PD-1/PDL-1 pathway, which is an inhibitor of immune activation (18). Therefore, if the PD-1/PDL-1 pathway is blocked pharmacologically, there may be enhanced anti-tumor response. In addition, studies have shown in other malignancies that increased tumor-infiltrating lymphocytes predict for better clinical outcomes (19).
Therefore, immunotherapy is a promising approach to combine with chemotherapy and radiation therapy in LA-NSCLC (20). A prior phase III randomized trial from the Chiba Cancer Center in patients with lung cancer who underwent resection investigated the role of IL-2 and lymphokine activated killer (LAK) cell adoptive immunotherapy in addition to cisplatin, vandesine, and mytomycin C (versus no additional therapy) and found improved overall survival (21). More modern immunotherapy trials are shown in Table 1, listing the different immunotherapy targets currently being evaluated in this setting. In addition to the targets listed, there are additional immunotherapy approaches planned but not yet underway for LA-NSCLC, including stimulating antigen-specific immune responses by vaccination, such as the MAGE-A3, belagenpumatucel-L, or TG4010 vaccines (22).
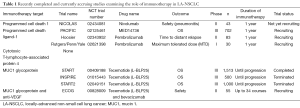
Full table
The START trial is the first to test immunotherapy in LA-NSCLC in a randomized, phase III setting (8). The drug studied in the START trial, tecemotide (L-BLP25), induces a T-cell response to the mucin 1 (MUC1) glycoprotein, which is abnormally glycosylated in NSCLC. The primary endpoint was overall survival, and block randomization was used to ensure similar baseline characteristics of both groups, including stage (IIIA versus IIIB), response to primary chemoradiotherapy (stable disease versus objective response), chemotherapy sequencing (concurrent or sequential with radiation therapy), and geographic region (North America and Australia versus Western Europe versus the rest of the world). A total of 1,513 patients were initially enrolled in a 2:1 randomization, and ultimately 829 in the tecemotide group and 410 in the placebo group were included in the modified intention-to-treat analysis, with this patient drop off largely due to a clinical hold of the drug by the study sponsor. The median overall survival for these arms were 25.6 and 22.3 months, respectively, but this was not statistically significant (adjusted HR, 0.88, 0.75–1.03, P=0.123). This effect, however, was statistically significant in patients receiving concurrent chemoradiation (30.8 vs. 20.6 months, adjusted HR 0.78, 0.64–0.95, P=0.016), the accepted standard of care for LA-NSCLC (7). The primary concern for toxicity with integrating immunotherapy with radiation therapy is pneumonitis, and dyspnea occurred in 5 and 4% and pneumonia in 2 and 3% of the groups, respectively. Overall, the drug was well-tolerated with no increase in the serious adverse events rate over placebo.
The positive results of the trial only in the concurrent but not sequential chemoradiation setting are not surprising, not only given that concurrent patients likely have better performance statuses and smaller tumors, but also given preclinical data suggesting that T-cell mediated lysis against MUC-1 is greater after concurrent therapy than either single therapy (23). In addition, the authors provided in the appendix preliminary data that the effect is greater with some chemotherapies over others (vinorelbine and taxanes versus etoposide). The START2 and INSPIRE trials investigating tecemotide alone have been terminated (11); the ECOG trial investigating tecemotide and bevacizumab is accruing, and we hope that this trial will stratify by chemotherapy regimen.
The potential of immunotherapy in LA-NSCLC lies largely in its ability to improve locoregional control or induce an abscopal effect of micrometastatic disease, and in doing so improve overall survival. The authors of the START trial should be commended for conducting the first phase III trial of immunotherapy maintenance in patients with LA-NSCLC and demonstrating safety and improved outcomes, at least in the subset of patients receiving concurrent chemoradiation. Despite the promising findings of this trial and shared optimism for future trials to prove to be efficacious, several questions regarding immunotherapy for LA-NSCLC remain. What are the appropriate immunotherapeutic agents to use? What is the optimal length of use (36% of START trial patients had >52 weeks of therapy)? Should these agents be given before, during, or after radiotherapy and/or chemotherapy? With the newfound use of immunotherapy as monotherapy following recurrence of LA-NSCLC after chemoradiation, and thus the increasing potential for study arm crossover, what are appropriate trial endpoints? Are outcomes dependent on molecular markers (PD-1, PD-L1)? Can immunotherapy be optimized to benefit patients potentially not fit for concurrent chemoradiation? What are the optimal cytotoxic chemotherapeutic agents to use with immunotherapy, and do some enhance the effects while others hinder response? And, lastly, which radiation therapy techniques—photon therapy versus proton therapy and conventionally fractionated radiotherapy versus stereotactic body radiation therapy (SBRT)—are optimally integrated with immunotherapies?
The START trial and others are using immunotherapy to take the brakes off of the host immune response in LA-NSCLC. Global investigators will now have to step on the gas to answer the many remaining questions regarding immunotherapy in LA-NSCLC.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Commentary commissioned by Guest Editor Hongcheng Zhu, MD, PhD (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [PubMed]
- Hoang T, Dahlberg SE, Schiller JH, et al. Randomized phase III study of thoracic radiation in combination with paclitaxel and carboplatin with or without thalidomide in patients with stage III non-small-cell lung cancer: the ECOG 3598 study. J Clin Oncol 2012;30:616-22. [PubMed]
- Vokes EE, Senan S, Treat JA, et al. PROCLAIM: A phase III study of pemetrexed, cisplatin, and radiation therapy followed by consolidation pemetrexed versus etoposide, cisplatin, and radiation therapy followed by consolidation cytotoxic chemotherapy of choice in locally advanced stage III non-small-cell lung cancer of other than predominantly squamous cell histology. Clin Lung Cancer 2009;10:193-8. [PubMed]
- Wozniak AJ, Moon J, Thomas CR Jr, et al. A Pilot Trial of Cisplatin/Etoposide/Radiotherapy Followed by Consolidation Docetaxel and the Combination of Bevacizumab (NSC-704865) in Patients With Inoperable Locally Advanced Stage III Non-Small-Cell Lung Cancer: SWOG S0533. Clin Lung Cancer 2015;16:340-7. [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [PubMed]
- Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:59-68. [PubMed]
- Kazandjian D, Khozin S, Blumenthal G, et al. Benefit-Risk Summary of Nivolumab for Patients With Metastatic Squamous Cell Lung Cancer After Platinum-Based Chemotherapy: A Report From the US Food and Drug Administration. JAMA Oncol 2016;2:118-22. [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]
- Daly ME, Monjazeb AM, Kelly K. Clinical Trials Integrating Immunotherapy and Radiation for Non–Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1685-93. [PubMed]
- Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003;170:6338-47. [PubMed]
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009;10:718-26. [PubMed]
- Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756-63. [PubMed]
- Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24:589-602. [PubMed]
- Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002;62:1462-70. [PubMed]
- Matsumura S, Demaria S. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiat Res 2010;173:418-25. [PubMed]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458-68. [PubMed]
- Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33:983-91. [PubMed]
- Simone CB 2nd, Burri SH, Heinzerling JH. Novel radiotherapy approaches for lung cancer: combining radiation therapy with targeted and immunotherapies. Transl Lung Cancer Res 2015;4:545-52. [PubMed]
- Kimura H, Yamaguchi Y. A phase III randomized study of interleukin-2 lymphokine-activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma. Cancer 1997;80:42-9. [PubMed]
- Iyengar P, Gerber DE. Locally advanced lung cancer: an optimal setting for vaccines and other immunotherapies. Cancer J 2013;19:247-62. [PubMed]
- Gelbard A, Garnett CT, Abrams SI, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res 2006;12:1897-905. [PubMed]


