
Clinical characteristics, surgical treatments, prognosis, and prognostic factors of primary tracheal cancer patients: 20-year data of the National Cancer Center, China
Introduction
Primary tracheal cancers are rare malignancies that account for only 2% of upper airway tumors. They represent 0.1–0.4% of all newly diagnosed cancers (1,2). The two main histological subtypes of tracheal cancer are adenoid cystic carcinoma (ACC) and squamous cell carcinoma (SCC) (2,3).
Patients with tracheal cancers usually present with upper airway symptoms such as wheezing, stridor, or hemoptysis (4). Due to low incidence and non-specificity of symptoms, tracheal cancers are usually diagnosed at a late stage, leading to poor patient prognosis, with the 5-year overall survival (OS) rate not exceeding 30% (1,3,5).
surgery is considered the mainstay of treatment for primary tracheal cancer (6,7). Other methods like endoscopic resection, radiotherapy, and chemotherapy also bring survival and palliative benefits, yet their roles in curative treatment remain controversial (8,9). The peculiar anatomy of tracheal cancers and the need for airway reconstruction make the surgery challenging.
To date, many reports on tracheal cancers have been limited to case reports or small case series (10-12). Studies of large cohorts are mostly based on public data sets (the Surveillance, Epidemiology, and End Results database and National Cancer Database) (2,3,5,7,13) or meta-analysis (6) and there are disparities among the findings of these reports. Some studies found that ACC was the most common subtype (6,7) while others identified SCC (2,3). SCC were found to dismal prognosis compared to other subtypes and He and colleagues reported other prognostic factors including age, tumor size and tumor extension (13). As to treatment of the disease, majority of the studies concluded that the use of surgery, especially curative surgery could provide better survival (3,5-7), yet the strategy of comprehensive treatment remains undetermined. Large scale case summaries will facilitate better understanding of the disease and improvements in diagnosis and treatment.
In the current study, we collected primary tracheal cancer patients who underwent surgical procedures in our center from January 2000 to December 2020. Their clinical, pathological, and surgery-related features were summarized and compared between the two major subtypes of ACC and SCC. Factors associated with their survival were also analyzed. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-258/rc).
Methods
The study is a cross-sectional study. This is a retrospective summary of case series that enrolled all primary tracheal cancer patients who had undergone surgical resection at the National Cancer Center, Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS) between January 2000 and December 2020. The electronic medical record system of our center was searched and all cases that matched the inclusion criteria were extracted and enrolled in our analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of CHCAMS (No. NCC3499). Individual consent for this retrospective analysis was waived.
Clinical information
Demographic, clinical, surgical, and pathological information was collected from patient medical records, surgical records, and pathological examination reports, which were included in the electronic medical record system. As the accurate length of tracheal resection (1–5 cm) was only recorded in some patients, it was not included in the current study for analysis. The histological findings of all enrolled patients were re-evaluated by two independent pathologists (XL-F and SS-S). Frozen section of the margins was performed during all the surgical procedures and resections were classified as follows: R0, no residual tumor; R1, microscopic residual tumor; and R2, macroscopic residual tumor. Pathological classification of primary tumor and lymph nodes was conducted as described in our previous studies (14,15). Simply, E1: primary tumor confined to the trachea or spread outside the trachea but not to adjacent organs; E2: primary tumor invading adjacent organs or other structures; N−: no lymph node metastasis; N+: positive lymph node metastasis; and NX: no lymph node dissection. Prognosis outcomes were obtained from follow-up outpatient clinic records or telephone interviews. Follow-up was done mainly by telephone interviews. For patients who had visited the outpatient clinic within 1 month from the analysis, the medical records were used to obtain the survival data. Patients with missing survival data were not included in the survival analysis.
Statistical analysis
All statistical analyses were performed using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA). Quantitative data were presented as the mean with standard deviation (SD) and compared by Student’s t-test. Categorical data were presented as a number with percentage and compared by chi-square test or Fisher’s exact test. The OS was defined as months from diagnosis or surgery to death and analyzed using Kaplan-Meier analysis. Univariable and multivariable Cox-regression analysis were applied to identify survival associated factors. All the tests were two-sided and P value <0.05 was considered statistically significant.
Results
Between January 2000 and December 2020, 128 patients with primary tracheal cancer underwent surgery in our center. Detailed patient information is presented in website: https://cdn.amegroups.cn/static/public/tlcr-22-258-1.xlsx.
Demographic and clinical characteristics
Firstly, we summarized the demographic and clinical features of the enrolled patients. Among the 128 participants, ACC was the most prevalent subtype (78/128, 60.9%), followed by SCC (24/128, 18.8%). Males represented 49.2% of the cohort and the average age was 49.4±13.6 years. About one-third of participants were smokers and dyspnea and cough were the most common chief complaints, accounting for 39.1% and 35.9%, respectively. Half of the tumors were located on the cervical part of the trachea, with the thoracic and carinal parts representing 41.4% and 8.6%, respectively. Two participants had received neoadjuvant therapy before surgery, and both were ACC patients (Table 1). Compared to ACC, SCC patients were significantly older (57.6±9.3 vs. 49.1±12.1 years, P=0.002), and had a higher proportion of males (75.0% vs. 39.7%, P=0.004) and smokers (75.0% vs. 25.6%, P<0.001). In addition, the percentages of patients with a history of cancer and comorbidities were significantly higher in SCC (Table 1). Compared to SCC, ACC was more frequently located in the cervical part of the trachea (50.0% vs. 33.3%, P=0.014), while the frequency of carinal tumor was higher in SCC (25.0% vs. 5.1%, P=0.014) (Table 1).
Table 1
| Feature | All (n=128)c | ACC (n=78) | SCC (n=24) | Others (n=26) | P valued |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 49.4 (13.6) | 49.1 (12.1) | 57.6 (9.3) | 43.9 (16.6) | 0.002 |
| Gender (male), n (%) | 63 (49.2) | 31 (39.7) | 18 (75.0) | 14 (53.8) | 0.004 |
| Tobacco use (yes), n (%) | 45 (35.2) | 20 (25.6) | 18 (75.0) | 7 (26.9) | <0.001 |
| Personal historya (yes), n (%) | 6 (4.7) | 1 (1.3) | 4 (16.7) | 1 (3.8) | 0.010 |
| Family historyb (yes), n (%) | 8 (6.3) | 4 (5.1) | 4 (16.7) | 0 | 0.086 |
| Comorbidities (yes), n (%) | 6 (4.7) | 11 (14.1) | 8 (33.3) | 6 (23.1) | 0.042 |
| Prime symptoms, n (%) | 0.377 | ||||
| Dyspnea | 50 (39.1) | 31 (39.7) | 7 (29.2) | 12 (46.2) | |
| Cough | 46 (35.9) | 29 (37.2) | 9 (37.5) | 8 (30.8) | |
| No symptom | 13 (10.2) | 8 (10.3) | 2 (8.3) | 3 (11.5) | |
| Hemoptysis | 10 (7.8) | 4 (5.1) | 3 (12.5) | 3 (11.5) | |
| Hoarseness | 8 (6.3) | 6 (7.7) | 2 (8.3) | 0 | |
| Dysphagia | 1 (0.8) | 0 | 1 (4.2) | 0 | |
| Tumor location, n (%) | 0.014 | ||||
| Cervical | 64 (50.0) | 39 (50.0) | 8 (33.3) | 17 (65.4) | |
| Thoracic | 53 (41.4) | 35 (44.9) | 10 (41.7) | 8 (30.8) | |
| Carinal | 11 (8.6) | 4 (5.1) | 6 (25.0) | 1 (3.8) | |
| Neoadjuvant therapy (yes), n (%) | 2 (1.6) | 2 (2.6) | 0 | 0 | 0.583 |
a, personal history of cancer; b, family history of cancer; c, all included ACC, SCC and other rare tracheal tumors; d, compared between ACC and SCC. SD, standard deviation; ACC, adenoid cystic carcinoma; SCC, squamous cell carcinoma.
Pathological findings
The number and percentage of all pathological subtypes are listed in Table S1. The average size of the tumors was 2.8±1.4 cm. Many tumors were E1 disease that did not invade adjacent organs (87, 68.0%) and 7.8% of participants had lymph node metastasis (Table 2). The participants with SCC had a significantly higher percentage of nodal metastasis than ACC patients (20.8% vs. 5.1%, P=0.036), while tumor size and extension were similar between the two subgroups (Table 2).
Table 2
| Features | All (n=128)a | ACC (n=78) | SCC (n=24) | Others (n=26) | P valueb |
|---|---|---|---|---|---|
| Tumor size (cm), mean (SD) | 2.8 (1.4) | 2.9 (1.2) | 2.8 (1.8) | 2.3 (1.4) | 0.847 |
| Tumor extension, n (%) | 0.470 | ||||
| E1 | 87 (68.0) | 47 (60.3) | 17 (70.8) | 23 (88.5) | |
| E2 | 41 (32.0) | 31 (39.7) | 7 (29.2) | 3 (11.5) | |
| Lymph node classification, n (%) | 0.036 | ||||
| N− | 75 (58.6) | 45 (57.7) | 14 (58.3) | 16 (61.5) | |
| N+ | 10 (7.8) | 4 (5.1) | 5 (20.8) | 1 (3.8) | |
| Nx | 42 (32.8) | 29 (37.2) | 5 (20.8) | 9 (34.6) |
a, all included ACC, SCC and other rare tracheal tumors; b, compared between ACC and SCC. SD, standard deviation; ACC, adenoid cystic carcinoma; SCC, squamous cell carcinoma.
Surgery-related features
We then examined the surgical procedures and surgery-related features of participants. Cervical incision and right thoracotomy were the two most common approaches used in nearly 90% of the surgical procedures. In summary, tracheal resection plus reconstruction was performed on 98 (76.6%) of the patients, among which 21 also received partial or total resection of the thyroid due to the thyroid invasion of primary tumors (Table 3). Carinal resection with neocarina was the third most common procedure, and the number of other procedures performed are also listed in Table 3. Over 60% of the surgical procedures had clear resection margins and lymph dissection was performed in 66.4%. The overall complication rate was 7.0% and 3 patients died of respiratory failure or cardiopulmonary arrest within 90 days after surgery, resulting in a perioperative death rate of 2.3%. After surgery, 63 (49.2%) of the participants received adjuvant therapy, the majority (56/63, 88.9%) of whom received radiotherapy (Table 3). In comparison, a cervical incision was used significantly more frequently in ACC (50% vs. 33.3%, P=0.018), while SCC had a higher proportion of sternotomy (12.5% vs. 2.6%, P=0.018). In terms of surgical procedures, tracheal resection plus reconstruction was performed on a significantly higher percentage of ACC patients (64.1% vs. 41.7%, P=0.011), In comparison, carinal resection with neocarina was used more frequently in SCC cases (20.8% vs. 9.0% P=0.011). The percentage of radical resection (R0) was significantly lower in ACC than in SCC surgical procedures (48.7% vs. 79.2%, P=0.030), while SCC surgical procedures had significantly higher complication rate (20.8% vs. 5.1%, P=0.031) (Table 3).
Table 3
| Features | All (n=128)a | ACC (n=78) | SCC (n=24) | Others (n=26) | P valueb |
|---|---|---|---|---|---|
| Incision, n (%) | 0.018 | ||||
| Cervical | 60 (46.9) | 39 (50.0) | 8 (33.3) | 13 (50.0) | |
| Right thoracotomy | 55 (43.0) | 33 (42.3) | 11 (45.8) | 11 (42.3) | |
| Sternotomy | 5 (3.9) | 2 (2.6) | 3 (12.5) | 0 | |
| Cervical + right thoracotomy | 4 (3.1) | 4 (5.1) | 0 | 0 | |
| Cervical + sternotomy | 3 (2.3) | 0 | 1 (4.2) | 2 (7.7) | |
| Left thoracotomy | 1 (0.8) | 0 | 1 (4.2) | 0 | |
| Surgical procedure, n (%) | 0.011 | ||||
| Tracheal resection + reconstruction | 77 (60.2) | 50 (64.1) | 10 (41.7) | 17 (65.4) | |
| Tracheal resection + thyroidectomy | 21 (16.4) | 11 (14.1) | 5 (20.8) | 5 (19.2) | |
| Carinal resection with neocarina | 13 (10.2) | 7 (9.0) | 5 (20.8) | 1 (3.8) | |
| Tracheal resection + total laryngectomy | 6 (4.7) | 6 (7.7) | 0 | 0 | |
| Enucleation | 5 (3.9) | 4 (5.1) | 1 (4.2) | 0 | |
| Tracheal resection + tracheostomy | 3 (2.3) | 0 | 0 | 3 (11.5) | |
| Carinal resection + lobar resection | 2 (1.6) | 0 | 2 (8.3) | 0 | |
| Left carinal pneumonectomy | 1 (0.8) | 0 | 1 (4.2) | 0 | |
| Resection margin, n (%) | 0.030 | ||||
| R0 | 79 (61.7) | 38 (48.7) | 19 (79.2) | 22 (84.6) | |
| R1 | 44 (34.4) | 36 (46.2) | 5 (20.8) | 3 (11.5) | |
| R2 | 5 (3.9) | 4 (5.1) | 0 | 1 (3.8) | |
| Lymph node dissection (yes), n (%) | 85 (66.4) | 49 (62.8) | 19 (79.2) | 17 (65.4) | 0.215 |
| Complications (yes), n (%) | 9 (7.0) | 4 (5.1) | 5 (20.8) | 1 (3.8) | 0.031 |
| Peri-operative death (yes), n (%) | 3 (2.3) | 1 (1.3) | 2 (8.3) | 0 | 0.137 |
| Adjuvant therapy (yes), n (%) | 63 (49.2) | 46 (59.0) | 12 (50.0) | 5 (19.2) | 0.485 |
| Adjuvant therapy, n (%) | 0.829 | ||||
| Radiotherapy | 56 (43.8) | 43 (55.1) | 11 (45.8) | 2 (7.7) | |
| Chemotherapy | 4 (3.1) | 2 (2.6) | 1 (4.2) | 1 (3.8) | |
| Chemoradiation | 3 (2.3) | 1 (1.3) | 0 | 2 (7.7) |
a, all included ACC, SCC and other rare tracheal tumors; b, compared between ACC and SCC. ACC, adenoid cystic carcinoma; SCC, squamous cell carcinoma.
Patient survival analysis
Further, we sought to explore the survival of tracheal cancer patients. The survival data of 12 participants were missing; thus, the analysis was conducted on 116 patients (ACC: 76; SCC: 20; others: 20) with a median follow-up time of 35.5 months. The median survival time of the 116 participants was 186 months with 25 found to be dead at the time of follow up. The 5- and 10-year survival rates were 84.5% and 63.7%, respectively. The median survival time of ACC and SCC patients were 198 and 130 months. The 5- and 10-year survival rates of ACC and SCC patients were 87.1%, 59.3%, and 53.0%, 52.9% respectively (Figure 1A,1B).
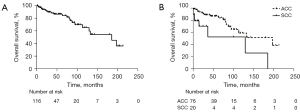
In order to exclude the bias caused by the low incidence pathological subtypes, we analyzed the prognostic factors among both ACC and SCC patients (n=96). Univariable and multivariable Cox-regression survival analysis was used to reveal the survival-related factors. The clinical and pathological covariables included gender, age (categorized by ≥50 or <50), comorbidities, personal history of cancer, tobacco use, resection margin, lymph node dissection, pathological subtype, tumor location, tumor extension, lymph node classification, postoperative complications, and adjuvant therapy. Univariable Cox-regression analysis was first performed and the covariables significantly associated with survival were included in the multivariable analysis. In univariable analysis, comorbidity, pathological subtype, tumor location, tumor extension, lymph node classification, and complications were significantly associated with the prognosis of the patients. Multivariable Cox-regression analysis showed that carinal tumor, E2 disease, lymph node metastasis, and postoperative complication were the independent predictors of poor patient OS (Table 4).
Table 4
| Features | Group | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (years) | ≥50/<50 | 1.354 (0.603–3.040) | 0.463 | |||
| Gender | Female/male | 1.209 (0.538–2.717) | 0.647 | |||
| Comorbidities | Y/N | 2.941 (1.173–7.369) | 0.021 | 1.300 (0.472–3.580) | 0.611 | |
| Personal history | Y/N | 1.793 (0.407–7.896) | 0.440 | |||
| Tobacco use | Y/N | 1.654 (0.722–3.789) | 0.234 | |||
| Resection margin | R1, R2/R0 | 1.279 (0.581–2.818) | 0.541 | |||
| Lymph node dissection | Y/N | 1.467 (0.583–3.690) | 0.415 | |||
| Subtype | SCC/ACC | 3.069 (1.296–7.268) | 0.011 | 1.270 (0.398–4.005) | 0.686 | |
| Tumor location | Carinal/cervical, thoracic | 5.903 (2.264–15.390) | <0.001 | 10.206 (2.840–36.683) | <0.001 | |
| Tumor extension | E2/E1 | 3.064 (1.241–7.563) | 0.015 | 8.870 (2.485–31.663) | 0.001 | |
| Lymph node classification | N+/N−, Nx | 10.043 (3.720–27.110) | <0.001 | 15.197 (3.844–60.076) | <0.001 | |
| Complications | Y/N | 4.694 (1.711–12.876) | 0.003 | 12.497 (2.824–55.306) | 0.001 | |
| Adjuvant therapy | Y/N | 0.963 (0.436–2.128) | 0.926 | |||
ACC, adenoid cystic carcinoma; SCC, squamous cell carcinoma; HR, hazard ratio; CI, confidence interval; Y, yes; N, no.
Discussion
In the current study, we have summarized the clinical and pathological features of primary tracheal cancer patients in a cohort of 128 cases who underwent surgical resection and assessed the prognostic factors for the patients. In our series ACC was the most common subtype of tracheal cancer, followed by SCC. SCC patients have a significantly higher rate of the carinal tumor, nodal metastasis, and postoperative complications. Survival analysis showed that carinal tumor, E2 disease, lymph node metastasis, and postoperative complications are significantly associated with unfavorable prognoses.
Due to the low incidence of the disease, the prevalence of different pathological subtypes of tracheal cancers remains undetermined. Different studies have reported different rankings between the two major subtypes, ACC and SCC, with some deeming SCC the most common type (1-3) and others nominating ACC (6,7). Our results showed that ACC was the most common subtype of resectable primary tracheal cancer, with ACC incidence being over 3 times that of SCC.
According to published studies, the 5-year survival rate of tracheal cancer patients is only around 30% (1,3), yet the cohort of 116 patients in our study had a 5-year survival rate of 84.5%. This is because all participants of our study had received surgery, most of which were radical resections. These results revealed that tracheal cancer patients could have a better prognosis given the opportunity of surgical resection, which agreed with previous studies (3,6,7). Thus, early detection and definitive surgery are fundamental to improving the prognosis of tracheal cancer patients.
Submucosal spread and perineural invasion commonly extend beyond the visible gross tumor of ACC, resulting in a high rate of positive resection margins despite aggressive surgery. In the current study, over a third of participants had positive resection margins, and the number was even higher among ACC patients, at over 50%. However, survival analysis found no significant difference in prognosis between patients with either clear or positive resection margins. Also, even though ACC patients had a significantly higher percentage of R1 and R2 resections than SCC patients, the OS of ACC patients was significantly longer than that of SCC patients. This could be explained by the fact that ACC typically has an indolent growth pattern, and also the majority of the R1 and R2 surgery patients have received radiotherapy after surgery, indicating the critical role of radiotherapy for patients with positive resection margins. These results also imply that even though complete resection cannot be achieved for some tracheal cancer patients, surgery should still be considered the priority of treatment, and radiotherapy should be recommended after surgery. For patients who underwent postoperative radiotherapy at our center, a mean target dose of 60 Gy was recommended, and the radiotherapy was often administered 6 weeks after surgery when a bronchoscopic assessment of anastomotic healing was performed. The recommended radiation field for surgically treated patients was 3 cm above and below the anastomosis.
In the survival analysis among ACC and SCC patients, comparison showed that SCC patients had significantly shorter OS than ACC patients, similar to previously published studies (2,3,13). However, the results of multivariable Cox-regression analysis revealed that pathological subtype was not among the independent prognostic factors. As a matter of fact, SCC patients were significantly older, had a significantly higher proportion of carinal tumor, lymph node metastasis, and postoperative complications, all of which may contribute to worse outcomes.
Published studies have reported the percentage of tracheal cancer patients who have received surgical procedures to be 20–40% (3,5-7). Thus our study was limited in that it only summarized the features of surgically treated patients. Also, follow-up information was unavailable for 12 participants and censored data was used for some of the patients in survival analysis, which could have led to bias.
To date, a comprehensive treatment strategy for tracheal cancer has not been established due to the rarity of the disease. Summaries of large patient cohorts are critical for expanding our knowledge of the disease. The National Cancer Center of China, is a national level center for cancer treatment in China, patients come from various regions around China. Thus, although this was a single-centre study, the cohort and results have valid representativeness and could contribute essential data and insight to this field of study.
Limitations
This study had some limitations. First of all, it was a retrospective study with a span of 20 years, which may affect the consistency of the treatment strategies. Second, a multiplicity of tumors with different prognosis were included in the current study, leading to complexity when describing patients’ prognosis. Third, only surgical patients were considered in the cohort, therefore the OS rate of the population was high. Moreover, as there was a high rate of R1 resection in the current study, it was hard to accurately definite the disease-free survival (DFS) of patients, and therefore DFS was not concluded in the current study.
Conclusions
The clinical, surgical, and pathological features of 128 surgically treated primary tracheal cancer patients were summarized and their survival-associated factors were investigated. Early diagnosis and complete resection are keys to better patient survival.
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: This work was supported by the National Key R&D Program of China (No. 2020AAA0109500), R&D Program of Beijing Municipal Education Commission (No. KJZD20191002302), Beijing Municipal Science & Technology Commission (No. Z191100006619119), and Beijing Natural Science Foundation (No. 7224342).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-258/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-258/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-258/coif). MVI serves as an unpaid editorial board member of Translational Lung Cancer Research from October 2021 to September 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of CHCAMS (No. NCC3499). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol 2011;34:32-7. [Crossref] [PubMed]
- Bhattacharyya N. Contemporary staging and prognosis for primary tracheal malignancies: a population-based analysis. Otolaryngol Head Neck Surg 2004;131:639-42. [Crossref] [PubMed]
- Agrawal S, Jackson C, Celie KB, et al. Survival trends in patients with tracheal carcinoma from 1973 to 2011. Am J Otolaryngol 2017;38:673-7. [Crossref] [PubMed]
- Macchiarini P. Primary tracheal tumours. Lancet Oncol 2006;7:83-91. [Crossref] [PubMed]
- Hararah MK, Stokes WA, Oweida A, et al. Epidemiology and treatment trends for primary tracheal squamous cell carcinoma. Laryngoscope 2020;130:405-12. [Crossref] [PubMed]
- Mallick S, Benson R, Giridhar P, et al. Demography, patterns of care and survival outcomes in patients with malignant tumors of trachea: A systematic review and individual patient data analysis of 733 patients. Lung Cancer 2019;132:87-93. [Crossref] [PubMed]
- Benissan-Messan DZ, Merritt RE, Bazan JG, et al. National Utilization of Surgery and Outcomes for Primary Tracheal Cancer in the United States. Ann Thorac Surg 2020;110:1012-22. [Crossref] [PubMed]
- Khaitan PG. Endoscopic Resection for Tracheal Adenoid Cystic Carcinoma? Is It Time to Change How We Practice? Ann Thorac Surg 2021;111:1093-4. [Crossref] [PubMed]
- Shah SA, Yang CJ, Berry MF. Endoscopic Instead of Surgical Resection of Tracheal ACC: Maybe, But Not So Fast… Ann Thorac Surg 2021;111:1094. Reply. [Crossref] [PubMed]
- Hazama K, Miyoshi S, Akashi A, et al. Clinicopathological investigation of 20 cases of primary tracheal cancer. Eur J Cardiothorac Surg 2003;23:1-5. [Crossref] [PubMed]
- Honings J, van Dijck JA, Verhagen AF, et al. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol 2007;14:968-76. [Crossref] [PubMed]
- Guo M, Peng G, Wei B, et al. Uniportal video-assisted thoracoscopic surgery in tracheal tumour under spontaneous ventilation anaesthesia. Eur J Cardiothorac Surg 2017;52:392-4. [Crossref] [PubMed]
- He J, Shen J, Huang J, et al. Prognosis of primary tracheal tumor: A population-based analysis. J Surg Oncol 2017;115:1004-10. [Crossref] [PubMed]
- Wang Y, Cai S, Gao S, et al. Tracheobronchial Adenoid Cystic Carcinoma: 50-Year Experience at the National Cancer Center, China. Ann Thorac Surg 2019;108:873-82. [Crossref] [PubMed]
- Wang Y, Cai S, Xue Q, et al. Treatment outcomes of patients with tracheobronchial mucoepidermoid carcinoma compared with those with adenoid cystic carcinoma. Eur J Surg Oncol 2020;46:1888-95. [Crossref] [PubMed]


