
Osimertinib in non-small cell lung cancer with uncommon EGFR-mutations: a post-hoc subgroup analysis with pooled data from two phase II clinical trials
Introduction
Treatment with tyrosine kinase inhibitors targeting epidermal growth factor receptor (EGFR-TKIs) is established as standard of care for patients with advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC). Deletions in exon 19 (del19) and the L858R point mutation in exon 21 constitute around 85% of the EGFR-mutations. The remaining 10–15% consist of a variety of mutations in exons 18 through 21, of which insertions in exon 20 (ex20ins) are the most frequent followed by the point mutations G719X (X representing A, C or S) in exon 18 either alone or in combination with others, S768I in exon 20 and L861Q in exon 21, respectively (1-3).
Multiple phase III trials have demonstrated the superiority of first and second generation EGFR-TKIs to chemotherapy for patients harbouring a sensitizing EGFR-mutation, with median progression-free survival (PFS) in the range of 9–15 months (4-7). Furthermore, the third generation TKI osimertinib, which is active against the sensitizing mutations and the T790M resistance mutation, had a median PFS of 18.9 months when used as first line treatment and 10.1 months in patients with acquired T790M-mediated resistance to previous EGFR-TKI treatment (8,9). However, most of the landmark studies of these agents only included patients with the common mutations del19 and L858R, and hence there are limited prospective data on the efficacy of these drugs in patients with uncommon EGFR-mutations. Whereas ex20ins are regarded as resistant to currently available EGFR-TKIs, retrospective studies have indicated some activity of first generation EGFR-TKIs in tumours harbouring the other uncommon mutations, albeit to a lesser degree than what is commonly reported for del19/L858R mutations (2,10-12). However, in a post-hoc analysis of 38 patients with G719X, L861Q or S768I from three clinical trials the overall response rate to the second generation EGFR-TKI afatinib was 71.1% and the median PFS was 10.7 months (13). Furthermore, the objective response rate (ORR) was 50% and the median PFS 8.2 months in a recent phase II trial with 36 EGFR-TKI naïve patients with uncommon mutations who were treated with osimertinib (14). Of the 36 patients in this study, 22 patients received osimertinib as first line therapy, whereas the remaining 14 had received at least one line of chemotherapy. Still, data on efficacy of these drugs in patients with the uncommon mutations are limited and prospective data are scarce.
We conducted two clinical phase II trials where EGFR-mutated patients received osimertinib as frontline treatment, and second or later line treatment, respectively. With the present analysis, we aimed to evaluate the activity of osimertinib in the subgroup of patients harbouring uncommon EGFR-mutations with pooled data from these two trials. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-995/rc).
Methods
We pooled individual data from a subgroup of patients with uncommon EGFR-mutations from two prospective clinical trials on osimertinib (NCT02504346 and NCT03804580). Both trials were phase II trials, had a single-arm design and were run in multiple centres in Northern Europe. Patients had advanced or metastatic NSCLC with an activating EGFR-mutation. One trial included patients previously treated with at least one EGFR-TKI; details of this trial have been published previously (15). The second trial included untreated patients. Patients in both trials were aged 18 years or older and provided written informed consent. In the previously treated patients, a re-biopsy was done after the last line of therapy and before commencement of the study treatment (osimertinib). For the untreated patients a biopsy done at diagnosis was accepted unless they had received adjuvant systemic cancer therapy after which a new biopsy was required. Testing for mutational status in tissue biopsies was done per local practice and included mainly real-time polymerase chain reaction (PCR) or next generation sequencing (NGS).
Blood samples were collected from each patient in the previously untreated cohort immediately before treatment initiation and after two weeks of treatment. Circulating tumour DNA (ctDNA) was isolated from blood samples and prepared for sequencing using the AVENIO ctDNA Surveillance Kit (Roche, Basel, Switzerland) as previously described (16). The samples were sequenced on NextSeq 500 High Output Lane (Illumina, San Diego, CA, USA).
All patients had an Eastern Cooperative Oncology Group (ECOG) status of 0–2, had adequate liver, renal and bone marrow function and had measurable disease as defined by RECIST v1.1. Imaging of all tumour lesions was done every 8 weeks the first 48 weeks and every 12 weeks thereafter. In the second line trial an MRI or CT scan of the brain was done if the patient had known or suspected brain metastases at baseline and was repeated throughout the study at the times of overall response assessments, whereas in the first line trial all patients were screened with a brain MRI at baseline and on every subsequent tumour assessment even in the absence of baseline brain metastases. All patients were treated with osimertinib 80 mg once daily until progressive disease by RECIST v1.1, or as long as they had clinical benefit as judged by the investigator. Dose reduction to 40 mg daily was allowed in case of significant toxicity. Reasons for discontinuation other than progressive disease were unacceptable toxicity, non-compliance or patient’s wish.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the national ethics committees in each participating country (Norway 2018/1028 and 2015/181, Sweden 2016/10-31/1 and 2019-02941, Finland 59/2015, Lithuania P-16-8 and P-18-85, Denmark H-15005843 and S-20180149) and informed consent was taken from all individual participants upon inclusion in the trials. The trials were registered at ClinicalTrials.gov (identifier: NCT02504346, NCT03804580). AstraZeneca and the South-Eastern Norway Regional Health Authority provided funding for the studies. The funding sources did not contribute to data collection, analyses, interpretation of the results or writing of the manuscript.
Outcome
The primary endpoint in the two clinical trials was ORR. Secondary endpoints included PFS, disease control rate (DCR), duration of response (DoR) and overall survival (OS). Other endpoints were intracranial progression-free survival (iPFS) and intracranial objective response rate (iORR). In this post-hoc, pooled analysis we assessed the efficacy endpoints of osimertinib in all patients with uncommon EGFR-mutations. Further, we divided the patients into two groups based on treatment line in which they received osimertinib (first line vs. pretreated) as this is clinically relevant. We also described efficacy according to the sensitizing mutations, with patients with G719X compound mutations in one group (“G719X compound group”), and patients with single mutations or combinations excluding G719X in the other group (“other mutation group”). As T790M is a resistance mutation rather than a sensitizing mutation, it was not regarded a compound to the other mutation when present. Furthermore, one patient was identified as having a single G719X mutation in tissue biopsy, but plasma NGS later revealed a rare partner mutation (L833V), which is of unknown clinical significance. As such, we categorized this patient in the “other mutation group”.
Statistical analysis
All time-to-event endpoints were analysed with the Kaplan-Meier method and subgroups compared with the log rank test. Two-way ANOVA test with Šidák correction was used to compare mutant ctDNA molecule concentration between groups. Two-sided P values of less than 0.05 were considered statistically significant. Confidence intervals were calculated with the exact method. All analyses were performed with IBM SPSS Statistics for Windows, Version 27.0 (Armonk, NY, USA: IBM Corp.).
Results
Patients
A total of 21 patients were included in the analysis, 10/199 patients from the second line study and 11/100 patients from the first line study. Patients were included from July 2015 to November 2018 and from December 2018 to June 2021 in the two trials, respectively. All patients received at least one dose of study medication and no patients were lost to follow up. The median age was 69 (range, 52–90) years, 81% were female, 19% were never-smokers and 24% had an ECOG-status of 2. At baseline, 38% of the patients had a G719X compound mutation with either S768I or L861Q as a partner mutation. L861Q and G719X as single mutations were found in 33% and 24% of the patients, respectively. In the previously treated patients, 30% had a T790M-mutation. For all pretreated patients, the median time on first or second generation EGFR-TKI before commencement of osimertinib was 18.0 months. The three T790M-positive patients had received a prior EGFR-TKI for 2.9, 16.1 and 34.7 months, respectively. Detailed baseline characteristics are presented in Table 1.
Table 1
| Baseline characteristics | Overall (n=21) | First line cohort (n=11) | Second or later line cohort (n=10) |
|---|---|---|---|
| Median age [range], years | 69 [52–90] | 75 [52–83] | 60.5 [52–90] |
| Sex | |||
| Male | 4 (19.0%) | 2 (18.2%) | 2 (20.0%) |
| Female | 17 (81.0%) | 9 (81.8%) | 8 (80.0%) |
| Smoking history | |||
| Never-smoker | 4 (19.0%) | 3 (27.3%) | 1 (10.0%) |
| Former smokeri | 15 (71.4%) | 6 (54.5%) | 9 (90.0%) |
| Current smokerii | 2 (9.5%) | 2 (18.2%) | 0 |
| ECOG status | |||
| ECOG 0–1 | 16 (76.2%) | 8 (72.7%) | 8 (80.0%) |
| ECOG 2 | 5 (23.8%) | 3 (27.3%) | 2 (20.0%) |
| Histology | |||
| Adenocarcinoma | 21 (100.0%) | 11 (100.0%) | 10 (100.0%) |
| EGFR-mutation at start osimertinib | |||
| G719X + S768I | 6 (28.6%) | 3 (27.3%) | 3 (30.0%)iii |
| G719X + L861Q | 2 (9.5%) | 1 (9.1%) | 1 (10.0%)iv |
| G719X | 5 (23.8%) | 2 (18.2%) | 3 (30.0%)v |
| L861Q | 7 (33.3%) | 5 (45.5%) | 2 (20.0%) |
| L861Q + ex20ins | 1 (4.8%) | 0 | 1 (10.0%) |
| Disease classification | |||
| Stage III | 1 (4.8%) | 0 | 1 (10.0%) |
| Stage IV | 20 (95.2%) | 11 (100.0%) | 9 (90.0%) |
| Extent of disease | |||
| Lung | 21 (100.0%) | 11 (100.0%) | 10 (100.0%) |
| Regional lymph nodes | 6 (28.6%) | 5 (45.5%) | 1 (10.0%) |
| Liver | 5 (23.8%) | 3 (27.3%) | 2 (20.0%) |
| Adrenal gland | 5 (23.8%) | 4 (36.4%) | 1 (10.0%) |
| CNS | 12 (57.1%) | 7 (63.6%) | 5 (50.0%) |
| Bone | 12 (57.1%) | 6 (54.5%) | 6 (60.0%) |
| No. of previous EGFR-TKI regimens | |||
| 1 | – | – | 9 (90.0%) |
| 2 | – | – | 1 (10.0%) |
| Previous EGFR-TKI therapy | |||
| First line | – | – | |
| Erlotinib | – | – | 8 (80.0%) |
| Gefitinib | – | – | 1 (10.0%) |
| Afatinib | – | – | 1 (10.0%) |
| Second line | – | – | |
| Erlotinib | – | – | 0 |
| Gefitinib | – | – | 0 |
| Afatinib | – | – | 1 (10.0%) |
| No. of other previous systemic anti-cancer treatmentsvi | |||
| 0 | – | – | 2 (20.0%) |
| 1 | – | – | 6 (60.0%) |
| 2 | – | – | 2 (20.0%) |
Data are presented as number (percentage). i, stopped smoking at least one year ago; ii, included stopped smoking the last year; iii, one T790M positive; iv, one T790M positive; v, one T790M positive; vi, systemic anticancer therapy for metastatic disease or adjuvant treatment ≤6 months before metastatic disease. ECOG, Eastern Cooperative Oncology Group; CNS, central nervous system.
Efficacy
Data cut-off was October 29, 2021. The ORR was 47.6% (95% CI: 25.7–70.2%) (Table 2 and Figure 1). In the first line cohort, the ORR was 63.6% (95% CI: 30.8–89.1%) and in the pretreated cohort 30.0% (95% CI: 6.7–65.2%). There were no complete responses. The DCR was 85.7% overall, and 100.0% and 70.0% in the first line and pretreated cohorts, respectively. The median DoR in the first line cohort was 12.1 months (95% CI: 0–29.2) vs. 7.8 months (95% CI: 4.2–11.4) in the pretreated cohort, P=0.602. We analysed response rates according to different EGFR-mutations (Table 2). For patients with a G719X compound mutation (n=8), the ORR was 62.5% (95% CI: 24.5–91.5%) and 38.5% (95% CI: 13.9–68.4%) in the group with other mutations (n=13). The DCR was 100.0% (95% CI: 63.1–100.0%) and 76.9% (95% CI: 46.2–95.0%) for the two groups, respectively. The median DoR was 12.4 months for the G719X-compound group vs. 3.8 months for the other mutations group, respectively, P=0.007.
Table 2
| Patient groups | Objective response, %, (95% CI) | Disease control, %, (95% CI) | DoR, months, (95% CI) |
|---|---|---|---|
| Overall (n=21) | 47.6 (25.7, 70.2) | 85.7 (63.7, 97.0) | 7.9 (0, 17.0) |
| 1st line cohort (n=11) | 63.6 (30.8, 89.1) | 100 (71.5, 100) | 12.1 (0, 29.2)§ |
| Pretreated cohort (n=10) | 30.0 (6.7, 65.2) | 70.0 (34.8, 93.3) | 7.8 (4.2, 11.4)§ |
| G719X compound mutations (n=8) | 62.5 (24.5, 91.5) | 100.0 (63.1, 100) | 12.4 (11.9, 12.9)* |
| G719X + S768I (n=5) | |||
| G719X + S768I + T790M (n=1) | |||
| G719X + L861Q (n=1) | |||
| G719X + L861Q + T790M (n=1) | |||
| Other mutations (n=13) | 38.5 (13.9, 68.4) | 76.9 (46.2, 95.0) | 3.8 (2.5, 4.1)* |
| G719X (n=4) | |||
| G719X + T790M (n=1) | |||
| L861Q (n=7) | |||
| L861Q + ex20ins (n=1) |
Note that the cohorts with different mutations are overlapping with the line of treatment cohorts. §, DoR first line vs. pretreated (P=0.602); *, DoR combination vs. other (P=0.007). DoR, duration of response.
Median PFS was 5.5 months (95% CI: 4.2–6.7) (Figure 2A). The median PFS was equal in the first line vs. pretreated cohort with 5.5 months in both groups, P=0.682 (Figure 2B). However, there was a statistically significant difference in PFS between the G719X compound mutation group and the other mutation group with a median PFS of 13.7 vs. 3.5 months, respectively, P=0.003 (Figure 2C).
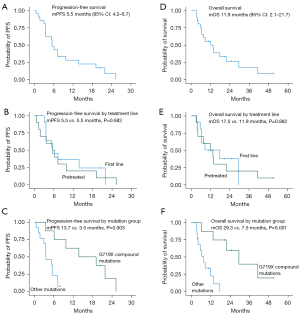
The median OS was 11.9 months (95% CI: 2.1–21.7) (Figure 2D). There was no statistically significant difference in OS between the first line and pretreated cohort with a median OS of 17.5 vs. 11.9 months, respectively (P=0.882) (Figure 2E). The median OS was longer in the compound mutations group with a median of 29.3 vs. 7.5 months in the group with other mutations, P=0.001 (Figure 2F).
Three of the patients harboured a T790M-mutation in addition to the uncommon mutation (Figures 1,3). We analysed the data with and without the T790M positive patients. The median PFS and median OS was identical whether the T790M positive were included or not (5.5 and 11.9 months, respectively).

Of 12 patients with brain metastases at baseline, 11 patients had brain scans available for review. Of these, eight patients had untreated brain metastases, two patients had received prior whole brain radiotherapy and one patient had been treated with stereotactic radiosurgery prior to inclusion. The iORR was 36.4% overall (Table 3) and the median iPFS was 6.1 months (95% CI: 1.3–10.8) (data not shown). In the first line cohort, the iORR was 42.3% vs. 25.0% in the pretreated cohort. The intracranial DCR was 100.0% in both subgroups. Further, in the G719X compound group, the iORR was 75.0% vs. 14.3% in the other mutation group. We did not calculate median iPFS in the subgroups due to small sample size.
Table 3
| CNS response | Overall* (n=11) | First line cohort (n=7) | Pretreated cohort (n=4) | G719X comb (n=4) | Other (n=7) |
|---|---|---|---|---|---|
| CR | 2 | 1 | 1 | 2 | 0 |
| PR | 2 | 2 | 0 | 1 | 1 |
| SD | 4 | 2 | 2 | 1 | 3 |
| Non-CR/non-PD | 3 | 2 | 1 | 0 | 3 |
| PD | 0 | 0 | 0 | 0 | 0 |
| iORR, % | 36.4 | 42.3 | 25.0 | 75.0 | 14.3 |
| iDCR, % | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
*, 6 patients had measurable disease, 5 patients only non-target lesions. CNS, central nervous system; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; iORR, intracranial objective response rate; iDCR, intracranial disease control rate.
ctDNA analysis
Sequencing of ctDNA from the first-line cohort led to identification of the identical uncommon EGFR-mutations in 9 out of the 11 patients that were identified in a tissue biopsy. Furthermore, in one patient with a single G719X mutation detected in tissue biopsy, ctDNA analysis identified an additional uncommon EGFR-mutation (L833V). The median number of all mutations identified was 3 mutations at baseline (range, 0–6) and 1 mutation after two weeks of treatment (range, 0–4). The number of mutant molecules per mL plasma was measured for all mutations, and the mean was calculated for each of the patients. All patients had a decrease in the mean number of mutant molecules per mL plasma after two weeks, except one patient with a small amount of ctDNA (8.64 to 13.01 mutant molecules per mL plasma), and one patient where no mutations were detected, neither before nor after treatment initiation. The decrease in ctDNA was detected in both the G719X compound group and in the group with a single uncommon EGFR-mutation (Figure 4). No significant difference was found between the number of mutant molecules in the plasma of the two groups (P=0.714 at baseline and P>0.999 at week 2) (data not shown). The EGFR-mutations in the G719X compounds had a similar allelic frequency (AF) in the baseline samples suggesting that they are in fact compound mutations present in the same cells (Table 4).
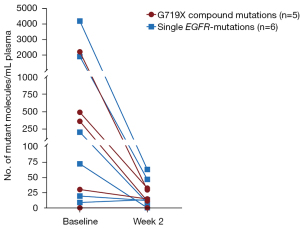
Table 4
| ID | Baseline (AF) | Difference between EGFR baseline mutations, % | Week 2 (AF) | ||
|---|---|---|---|---|---|
| G719X | Other EGFR mutation | G719X | Other EGFR mutation | ||
| 1 | 22.98 | 22.01 (S768I) | 4.22 | 0.21 | – |
| 2 | 7.77 | 8.62 (S768I) | 9.86 | – | – |
| 3 | – | – | – | – | – |
| 4 | 2.77 | 2.25, 0.11 (L861Q, ex19del) | 18.77 | – | 0.15 (L861Q) |
| 5 | 0.53 | 0.40 (L833V) | 24.53 | 0.22 | 0.12 (L833V) |
AF, allelic frequencies.
Safety
All patients reported at least one adverse event, with decreased appetite (13/21), nausea (10/21), rash (9/21) and paronychia (9/21) as the most common. The majority of the adverse events were of grade 1–2. Grade 3 or higher adverse events regardless of relation to study treatment were seen in 11/21 patients. Among these, three cases of pulmonary embolism, two cases of nausea and one case of erythema multiforme were considered as possibly treatment related (all grade 3). One patient discontinued osimertinib because of an adverse event (erythema multiforme). There were two deaths other than of progressive disease; one patient died of possible treatment-related pneumonitis and one patient died of cholecystitis not related to study treatment.
Discussion
Osimertinib has been shown to be superior to first generation EGFR-TKIs in patients with common EGFR-mutations (8,17) and to chemotherapy in patients with the T790M resistance mutation in addition to del19/L858R (9). However, although preclinical data suggest that osimertinib is active also in tumours with uncommon mutations (18), there are limited prospective clinical data to support this. In this post-hoc pooled analysis, we demonstrated that osimertinib has clinical activity in patients with uncommon mutations, with an overall ORR of 48% and DCR of 86%, respectively. Furthermore, the ORR of 64% (95% CI: 31–90%) in the first line cohort was consistent with the ORR (50%, 95% CI: 33–67%) in a Korean phase II-trial of patients treated in the first line setting (14). Although the ORR was lower in the second line cohort (30%), there was a clinically meaningful DCR of 70%. The DoR was similar, and clinically significant in both groups.
Despite the encouraging ORR, the median PFS of 5.5 months was modest and similar to that reported by Cho et al. (14) (8.2 months). Interestingly, the median PFS was identical in the first and second line cohorts and close to what we have previously found in the 52 T790M-negative patients in the overall patient population in the second line study (median PFS 5.1 months) (15). However, across all the efficacy parameters, there was a more favourable outcome for patients with G719X compound mutations than for patients with other mutations, which to our knowledge has not been described previously. For instance, there was a large and highly statistically significant difference in OS (median 29.3 vs. 7.5 months, P=0.001). In a retrospective observational study of patients with uncommon EGFR-mutations treated with gefitinib or erlotinib, Chiu et al. (10) demonstrated that the PFS was significantly longer for those with compound mutations than for single mutations (median PFS 11.9 vs. 6.5 months, P=0.010, respectively). Similar results were also reported in a Dutch study, indicating that uncommon compound mutations are more responsive to early generation EGFR-TKIs than single uncommon mutations (19). Furthermore, recent data indicate that response to EGFR-TKIs depends on mutational subgroups, and that osimertinib may differ from other EGFR-TKIs with respect to inhibition of the atypical mutation subtypes (20). ctDNA analysis showed that mutations within the G719X compound had a similar AF, which indicates that the two EGFR-mutations are in fact compound mutations existing in the same clone. This could possibly explain the favourable PFS and OS, however, the effect of double mutations on the structure-functional characteristics of the EGFR mutants is to our knowledge not known.
When looking at the number of ctDNA mutant molecules per mL plasma we found no significant difference between the G719X compound group and the group with a single EGFR-mutation. However, we saw a reduction in the mutant molecule concentration within the first two weeks of treatment, independent of mutational group. This ctDNA decrease may be a sign of early response to treatment. A study by Ebert et al. (21) showed that clearing of the ctDNA in plasma was correlated with both PFS and OS, independent of the rapidity of the clearing. We suggest that the observed decrease in ctDNA after two weeks of treatment illustrates ongoing clearing. Our results may indicate that uncommon EGFR-mutations, and especially compound mutations involving G719, render the kinase sensitive to osimertinib. Further studies are needed to explore whether these observations are due to inherent biological differences leading to a more indolent course of disease for the compound mutations, or due to a better treatment effect.
Of note, only 30% of the subset of patients with uncommon mutations who had developed resistance to an EGFR-TKI prior to treatment with osimertinib had detectable T790M. Despite the limited sample size in our material, this finding is in line with a recent retrospective study of patients with disease progression on EGFR-TKIs, where there was a significantly lower incidence of T790M in 27.1% of 48 patients with uncommon mutations vs. 55.2% and 37.2% in patients with del19 and L858R, respectively (22). Furthermore, studies have demonstrated that a longer duration of exposure to first generation EGFR-TKIs is associated with a higher incidence of T790M (23,24). Uncommon mutations have been reported to be less sensitive to first generation EGFR-TKIs with shorter PFS than the common mutations (2,10-12). However, of the three T790M-positive patients in our material, only one patient had a duration of EGFR-TKI prior to osimertinib that was longer than median (34.7 months, median 18.0 months) and one patient had short time on first generation TKI (2.9 months). Hence, whether fewer cases of T790M-mediated resistance are a consequence of shorter treatment time on first generation drugs remains uncertain.
Osimertinib is central nervous system (CNS)-penetrant and studies have shown a high activity in patients with common mutations and brain metastases (25,26). In the present analysis, the iORR was 36% and the iDCR 100%, demonstrating intracranial effect of osimertinib in the case of uncommon mutations as well. Furthermore, as for the overall efficacy endpoints, the iORR was markedly higher in patients with G719X compound mutations than for the other mutations (75% vs. 14%), indicating that the favourable outcome observed for G719X compound mutations also applies in the presence of brain metastases.
There are some limitations to this study. First, the number of patients is small precluding conclusive results based on this study alone. However, given the rarity of these mutations, it is challenging to perform large scale studies and, as such, all patient samples will add to the knowledge on the subject. Also, an ongoing Japanese phase II-study (jRCTs071200002) of osimertinib for previously untreated uncommon EGFR mutant NSCLC, which aims to include 40 patients, will be an important contribution to this (27). Second, because the second line trial included patients regardless of T790M-status (15), the pre-treated cohort consists of patients with and without T790M. We have therefore presented individual response data and performed extra analysis of PFS and OS which indicate similar efficacy regardless of T790M status.
In summary, we demonstrated that osimertinib exerts activity in patients with uncommon EGFR-mutations, both in treatment naïve patients and after progression on first or second generation EGFR-TKIs. The uncommon mutations are a heterogeneous group of mutations of which compound mutations with G719X seem to be most sensitive to treatment whereas single mutations including G719X alone are less responsive.
Acknowledgments
We would like to express our gratitude towards the patients and their families for contributing to this study. We also thank the clinical and research staff at all the participating sites for the collaboration, which made this work possible.
Funding: This work was supported by AstraZeneca (Nos. ESR-14-10267 and D5161C00007) and the South-Eastern Norway Regional Health Authority (Nos. 2021012 to IJZE and 2018049 to OTB).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-995/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-995/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-995/coif). IJZE has received honoraria for lectures or advisory boards for Novartis and Boehringer-Ingelheim. ÅH has received honoraria for lectures or advisory boards to her institution, from AstraZeneca, Takeda, Pfizer, Bayer, BMS and Roche. SC has received honoraria for lectures for Pfizer, Roche and AstraZeneca. JK has received grants from AstraZeneca and Boehringer-Ingelheim and honoraria for lectures or advisory boards from AstraZeneca, BMS, Boehringer-Ingelheim, MSD, Amgen, Eli Lilly, Sanofi and Roche. BHG has received honoraria for lectures and advisory boards for AstraZeneca, Pfizer, Takeda, Roche, MSD and Janssen. OTB has received honoraria for lectures or advisory boards from AstraZeneca, Roche, Novartis, Eli Lilly, Pfizer, Sanofi, Bayer, MSD, BMS, Boehringer-Ingelheim and grants from Astra Zeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the national ethics committees in each participating country (Norway 2018/1028 and 2015/181, Sweden 2016/10-31/1 and 2019-02941, Finland 59/2015, Lithuania P-16-8 and P-18-85, Denmark H-15005843 and S-20180149) and informed consent was taken from all individual participants upon inclusion in the trials. The trials were registered at ClinicalTrials.gov (identifier: NCT02504346, NCT03804580).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci 2016;107:1179-86. [Crossref] [PubMed]
- Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 2014;25:126-31. [Crossref] [PubMed]
- Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J Thorac Oncol 2018;13:1560-8. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, simertini phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a simertini, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Chiu CH, Yang CT, Shih JY, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol 2015;10:793-9. [Crossref] [PubMed]
- Yamada Y, Tamura T, Yamamoto Y, et al. Treatment of Patients With Non-small-cell Lung Cancer With Uncommon EGFR Mutations in Clinical Practice. Anticancer Res 2020;40:5757-64. [Crossref] [PubMed]
- Watanabe S, Minegishi Y, Yoshizawa H, et al. Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol 2014;9:189-94. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Cho JH, Lim SH, An HJ, et al. Osimertinib for Patients With Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J Clin Oncol 2020;38:488-95. [Crossref] [PubMed]
- Eide IJZ, Helland Å, Ekman S, et al. Osimertinib in T790M-positive and -negative patients with EGFR-mutated advanced non-small cell lung cancer (the TREM-study). Lung Cancer 2020;143:27-35. [Crossref] [PubMed]
- Clement MS, Ebert EBF, Meldgaard P, et al. Co-occurring MET Amplification Predicts Inferior Clinical Response to First-Line Erlotinib in Advanced Stage EGFR-Mutated NSCLC Patients. Clin Lung Cancer 2021;22:e870-7. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Floc’h N, Lim S, Bickerton S, et al. Osimertinib, an Irreversible Next-Generation EGFR Tyrosine Kinase Inhibitor, Exerts Antitumor Activity in Various Preclinical NSCLC Models Harboring the Uncommon EGFR Mutations G719X or L861Q or S768I. Mol Cancer Ther 2020;19:2298-307. [Crossref] [PubMed]
- Kuiper JL, Hashemi SM, Thunnissen E, et al. Non-classic EGFR mutations in a cohort of Dutch EGFR-mutated NSCLC patients and outcomes following EGFR-TKI treatment. Br J Cancer 2016;115:1504-12. [Crossref] [PubMed]
- Robichaux JP, Le X, Vijayan RSK, et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature 2021;597:732-7. [Crossref] [PubMed]
- Ebert EBF, McCulloch T, Hansen KH, et al. Clearing of circulating tumour DNA predicts clinical response to first line tyrosine kinase inhibitors in advanced epidermal growth factor receptor mutated non-small cell lung cancer. Lung Cancer 2020;141:37-43. [Crossref] [PubMed]
- Yang S, Mao S, Li X, et al. Uncommon EGFR mutations associate with lower incidence of T790M mutation after EGFR-TKI treatment in patients with advanced NSCLC. Lung Cancer 2020;139:133-9. [Crossref] [PubMed]
- Matsuo N, Azuma K, Sakai K, et al. Association of EGFR Exon 19 Deletion and EGFR-TKI Treatment Duration with Frequency of T790M Mutation in EGFR-Mutant Lung Cancer Patients. Sci Rep 2016;6:36458. [Crossref] [PubMed]
- Kuiper JL, Heideman DA, Thunnissen E, et al. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung Cancer 2014;85:19-24. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. J Clin Oncol 2018;36:3290-7. [Crossref] [PubMed]
- Wu YL, Ahn MJ, Garassino MC, et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol 2018;36:2702-9. [Crossref] [PubMed]
- Okuma Y, Shimokawa M, Hashimoto K, et al. Uncommon EGFR mutations conducted with osimertinib in patients with NSCLC: a study protocol of phase 2 study (UNICORN/TCOG1901). Future Oncol 2022;18:523-31. [Crossref] [PubMed]



