
Phase II study of nanoparticle albumin-bound paclitaxel monotherapy for relapsed non-small cell lung cancer with patient-reported outcomes (NLCTG1302)
Introduction
Recent advances in molecular-targeted therapies and immunotherapies using immune checkpoint inhibitors (ICIs) have emerged for the treatment of non-small cell lung cancer (NSCLC). These therapies have caused a paradigm shift in the treatment of NSCLC and provided long-term disease control in some patients. However, most patients acquire resistance to these therapies and subsequently require chemotherapy with cytotoxic agents. Cytotoxic chemotherapy is still an important modality in the treatment of lung cancer, and there is a need to increase treatment options in clinical practice.
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a nano-particulate agent that binds to human serum albumin (1). It is expected to potentiate clinical feasibility due to the absence of allergy for solutions and catalysts, and clinical efficacy due to high distribution in tissues (2). A randomized phase III trial was conducted to compare response rate of nab-paclitaxel plus carboplatin (CBDCA) with solvent-based paclitaxel plus CBDCA for initial treatment of NSCLC (the CA031 study). Nab-paclitaxel plus CBDCA showed a favorable response rate of 33% without severe neurotoxicity (3). Following these findings, a prospective study of nab-paclitaxel monotherapy for pretreated NSCLC was conducted and showed promising response rates ranging from 7.3–31.7% (4-11). In addition, the first phase III trial was conducted to evaluate the efficacy of nab-paclitaxel monotherapy in comparison with docetaxel (DTX) for previously treated patients with advanced NSCLC (J-AXEL) (12). Progression-free survival (PFS) was significantly longer in the nab-paclitaxel group than in the DTX group: median of 4.2 [95% confidence interval (CI): 3.9–5.0] vs. 3.4 (95% CI: 2.9–4.1) months. The overall response rate (ORR) was 15.4% (95% CI: 10.9%–20.7%) in the DTX group and 29.9% (95% CI: 24.0–36.2%) in the nab-paclitaxel group (P=0.0002). Because of these results, nab-paclitaxel is now considered a promising treatment option.
Chemotherapy-induced peripheral neuropathy (CIPN) is a common adverse event associated with taxane-based chemotherapy, including nab-paclitaxel. CIPN includes awareness of paresthesia and hypoesthesia, such as burning, numbness, ache, and penetrating pain. CIPN affects quality of life (QOL) and activities of daily living and also causes discontinuation, delay, and reduction of antitumor treatment (13). The percentage of patients who experienced severe grade or all-grade neurotoxicity was reported as 3% or 46%, respectively, in the CBDCA plus nab-paclitaxel group in the CA031 study. In the Japanese subgroup analysis in the CA031 study, 64% of patients experienced neurotoxicity (14). Thus, CIPN remains a problematic adverse event for patients who are treated with nab-paclitaxel based chemotherapy.
Objective assessment using the Common Terminology Criteria for Adverse Events (CTCAE) by primary physicians has commonly been used to assess adverse events in clinical trials (15). However, it has been pointed out that CTCAE may not be able to accurately assess a patient’s condition because the evaluation is at the discretion of the physician in charge (16). In particular, the assessment of CIPN using CTCAE may be considered insufficient because it is usually difficult for physicians to accurately and objectively assess the symptoms of neurotoxicity (17). The patient-reported outcome (PRO) is a report directly obtained from a patient without interpretation by health professionals (18). The importance of PRO has been increasing in CIPN assessment because these kinds of assessment tools can directly reflect a patient’s symptoms and provide valuable information in clinical practice. The Patient Neurotoxicity Questionnaire (PNQ) score is a validated PRO tool used to assess CIPN (19). In this study, we investigated numbness using the PNQ score system as an additional measure and evaluated the effects of peripheral neuropathy treatment on QOL.
Using this approach, we conducted a prospective, multicenter, single-arm, phase II trial to evaluate the efficacy and safety of nab-paclitaxel monotherapy in previously treated patients with advanced NSCLC. The neurotoxicity of nab-paclitaxel was also assessed as a secondary objective using the CTCAE scale and PNQ score system. We present the following article in accordance with the TREND reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-89/rc).
Methods
Study design
This clinical trial [Niigata Lung Cancer Treatment Group (NLCTG) 1302] was an open-label, multicenter, single-arm, phase II study involving 14 institutions in Niigata, Japan. The study was performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. The study was approved by the Ethics Committee of Niigata University (No. NH25-006) and informed consent was taken from all the patients. This study was started on November 1, 2013, and finished on September 31, 2016. The study was registered with the University Hospital Medical Information Network (UMIN) with the clinical trial number UMIN000012343.
Patients
Eligible patients were histologically or cytologically proven to have unresectable advanced NSCLC and recurrent or refractory disease after one or two cytotoxic chemotherapy regimens. Prior adjuvant chemotherapy was permitted if it was completed 12 months before enrollment in the study. An additional line of tyrosine kinase inhibitor therapy in patients with known epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) translocation was allowed. Criteria for enrolment in the study included: a performance status (PS) of 0 to 2 on the Eastern Cooperative Oncology Group (ECOG), age ≥20, a life expectancy of 12 weeks or more, adequate bone marrow reserve (absolute neutrophil count ≥1,500/mm3, platelet count ≥100,000/mm3), hemoglobin ≥9.0 g/dL, normal liver function (total serum bilirubin ≤2.0 mg/dL, aspartate transaminase and alanine transaminase ≤100 IU/dL), normal renal function (serum creatinine ≤1.2 mg/dL), and SpO2 ≥90%. Patients were excluded from the study if they had greater than grade 1 neuropathy according to CTCAE v4.0, or a history of allergy or hypersensitivity to the study drugs.
Treatment plan
The patients received 100 mg/m2 nab-paclitaxel intravenously on days 1, 8, and 15, every 4 weeks. Cycles were repeated until disease progression, unacceptable toxicity, or until the patient or investigator requested therapy discontinuation. Restarting was approved when adequate organ function was recovered and fulfilled the following criteria: neutrophil count ≥1,500/mm3, platelet count ≥100,000/mm3, ECOG PS ≤2, grade of any non-hematologic toxicity <2, and no severe infection. Before the administration of nab-paclitaxel on days 8 and 15, a neutrophil count ≥1,000/mm3 and a platelet count ≥75,000/mm3 were required. The dose was reduced to a minimum of 60 mg/m2 in cases of grade 4 thrombocytopenia, febrile neutropenia, and grade 3 or 4 increases in liver enzyme levels. Modification of the treatment schedule was allowed to maintain tolerability of treatment. This schedule modification was performed when the criteria for reducing the dose and drug administration were also not met. The details of the criteria for modifying the treatment schedule are shown in Figure S1.
Statistical analysis
The primary endpoint was ORR, assessed by investigator’s review. The secondary endpoints were PFS, overall survival (OS), disease control rate (DCR) toxicity profiles, and PRO using the PNQ score. Response rate of 2nd line or 3rd line monotherapy for NSCLC was 5–8% (20-22), and threshold response rate was set at 7%. Expected response rate was set at 17%, 10% higher than that of monotherapy. Using Simon’s two-stage design, with α=0.05 and β=0.20, we estimated that a sample size of 35 and 30 patients was required in stages one and two, respectively. The hypothesis that this treatment exceeds the threshold response rate of 7% would have been rejected if the number of responding patients was less than one in stage one, a finding that would have resulted in early termination of the study. Because this was not the case, stage two, with 65 patients, was allowed to begin. This study was considered positive if nine or more patients responded in this stage. PFS was defined as the time from the start of treatment to the date of disease progression or death. OS time was defined as the time from the date of the start of treatment to the date of death or last contact. Patients who could not be followed up were censored on the day survival was confirmed before it became impossible to pursue. Time-to-event distributions were estimated using the Kaplan-Meier method. Statistical analysis was performed using the JMP 10 software (SAS Institute, Cary, NC, USA).
PRO assessment
We investigated numbness using PNQ score to evaluate QOL. The PNQ is a simple questionnaire that assesses sensory and motor neuropathies. The severity of each item was evaluated using a five-point scale, ranging from grade A (no neuropathy) to grade E (very severe neuropathy). The answer options were coded from 0 to 4, with a higher score indicating more severe CIPN. These scores were obtained at baseline and every two cycles. We evaluated the course of the sensory and motor peripheral neuropathies over time. To examine clinical responsiveness according to treatment, PNQ scores and CTCAE were evaluated using linear time-trend tests. We also assessed the concordance between PNQ scores and CTCAE by comparing the absolute score distribution for each severity grade between the patient-based PNQ and physician-based CTCAE, using the weighted kappa coefficient. The categories used for interpreting kappa values were those previously proposed by Landis and Koch (23); kappa <0.00, poor; 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.00, almost perfect.
Results
Patient characteristics
From September 2013 to September 2016, 65 patients were enrolled from 14 participating institutions in Niigata. Table 1 shows the characteristics of the 65 eligible patients. There were 50 male (77%) and 15 female (23%) patients, with a median age of 69 (range, 40–85) years. All patients were Asian. Most patients (94.3%) had an acceptable ECOG PS score of 0–1. Histology of the patients was as follows: 43 (66.1%) had adenocarcinoma, 20 (30.8%) had squamous cell carcinoma, and 2 (3.1%) had adenosquamous cell carcinoma. Forty-one patients (63.1%) received nab-paclitaxel as a second-line therapy, and 24 (36.9%) received it as a third-line therapy. The details of regimens for first- and second-line treatments are summarized according to histological types (Tables S1-S3). Eight patients with adenocarcinoma had EGFR mutations (deletion of exon 19 or L858R), one patient with squamous cell carcinoma had a deletion of exon 19, and one patient with adenocarcinoma had a c-ros oncogene 1 (ROS1) rearrangement.
Table 1
| Characteristics | N | % |
|---|---|---|
| Age (years), median [range] | 69 [40–85] | |
| Gender | ||
| Male | 50 | 77.0 |
| Female | 15 | 23.0 |
| Clinical stage | ||
| IIIB | 5 | 7.7 |
| IV | 47 | 72.3 |
| Post-operative recurrence | 9 | 13.8 |
| Post-chemoradiation recurrence | 4 | 6.2 |
| Histology | ||
| Adenocarcinoma | 43 | 66.1 |
| Squamous cell carcinoma | 20 | 30.8 |
| Adenosquamous | 2 | 3.1 |
| ECOG PS | ||
| 0 | 8 | 22.9 |
| 1 | 25 | 71.4 |
| 2 | 2 | 5.7 |
| Smoking history | ||
| Never | 15 | 23.0 |
| Current/former | 50 | 77.0 |
| Driver gene mutations | ||
| EGFR | 9 | 13.8 |
| ROS1 | 1 | 1.5 |
| None/unknown | 55 | 84.6 |
| No. of prior treatment regimen | ||
| 1 | 41 | 63.1 |
| 2 | 24 | 36.9 |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; EGFR, epidermal growth factor receptor; ROS1, c-ros oncogene 1.
Treatment delivery
The median number of treatment cycles was 4 (range, 1–20), with 14 patients (21.5%) receiving at least six. The primary reasons for dose reduction were neutropenia and febrile neutropenia. Overall, 10 patients (15.3%) required dose reduction and 19 (29.2%) required schedule modification. Two patients required dose reduction and one patient discontinued treatment because of peripheral neuropathy.
Efficacy results
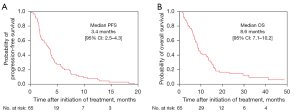
All 65 patients were eligible for efficacy analysis. Twelve patients (18.5%) attained a partial response (PR), and no patient attained a complete response (CR). Twenty-eight patients (43.1%) had stable disease (SD) and 25 (38.4%) had progressive disease (PD). The ORR and DCR were 18.5% (95% CI: 10.9–29.6%) and 61.5% (95% CI: 47.9–52.1%), respectively. In the final analysis, disease progression events were observed in all evaluable patients and survival events occurred in 59 patients. The median PFS was 3.4 (95% CI: 2.5–4.3) months (Figure 1A), and the median OS was 8.6 (95% CI: 7.1–10.2) months (Figure 1B). Subgroup analysis was performed regarding ORR and PFS in patients with or without squamous histology, prior DTX treatment, prior pemetrexed (PEM) treatment, prior gemcitabine treatment, and driver mutations (Table S3). In patients with squamous histology and prior gemcitabine treatment, there were statistically significant differences in PFS (P=0.0346) and ORR (P=0.0235), respectively. There was no statistical difference in the therapeutic effect with other covariant factors (Table S3). Multiplicity must be considered while interpreting this result. However, the effect of nab-paclitaxel tended to be higher in patients with squamous cell carcinoma in the phase III study (12). Differences in the effects of nab-paclitaxel depending on histology have been shown in multiple studies and may be applicable to clinical practice.
Safety
The hematologic and non-hematologic toxicity profiles of the 65 patients are listed in Table 2. The most common hematologic grade ≥3 treatment-related adverse events were neutropenia (30.8%), leukopenia (23.1%), and anemia (1.5%). The most common non-hematologic grade ≥3 treatment-related adverse events were infections (7.7%), hyponatremia (4.6%), alanine aminotransferase (ALT) increase (1.5%), and fatigue (1.5%). Grade 3 febrile neutropenia was observed in 4 patients (6.2%). Drug-induced pneumonitis occurred in one patient (1.5%). Treatment-related death occurred in one patient because of bronchopulmonary hemorrhage. Grade 1–2 peripheral sensory or motor neuropathy was observed in 31 (47.7%) and nine (13.8%) patients, respectively. Grade >3 peripheral sensory or motor neuropathy was not observed.
Table 2
| Adverse events | All grade | ≥ Grade 3 | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Hematological toxicities | |||||
| WBC decreased | 38 | 58.4 | 15 | 23.1 | |
| Neutropenia | 34 | 52.3 | 20 | 30.8 | |
| Anemia | 41 | 63 | 1 | 1.5 | |
| Thrombocytopenia | 3 | 4.6 | 0 | 0 | |
| Febrile neutropenia | 4 | 6.2 | 4 | 6.2 | |
| Non-hematological toxicities | |||||
| Appetite loss | 20 | 30.8 | 0 | 0 | |
| Constipation | 15 | 23.1 | 0 | 0 | |
| Fatigue | 26 | 40 | 1 | 1.5 | |
| Stomatitis | 10 | 15.4 | 0 | 0 | |
| Infection | 7 | 10.8 | 5 | 7.7 | |
| Interstitial lung disease | 0 | 0 | 1 | 1.5 | |
| Alopecia | 34 | 52.3 | 0 | 0 | |
| Peripheral sensory neuropathy | 31 | 47.7 | 0 | 0 | |
| Peripheral motor neuropathy | 9 | 13.8 | 0 | 0 | |
| Myalgia | 11 | 16.9 | 0 | 0 | |
| Liver dysfunction | 20 | 30.8 | 1 | 1.5 | |
| Hyponatremia | 18 | 27.7 | 3 | 4.6 | |
| Bronchopulmonary hemorrhage | 0 | 0 | 1 | 1.5 | |
CTCAE, Common Terminology Criteria for Adverse Events; WBC, white blood cell.
The PRO assessment
A total of 57 (88%) PNQ scores were evaluable at baseline, and 46 (78%), 29 (88%), 12 (67%), and 7 (70%) were evaluable before cycles 2, 4, 6, and 8, respectively. The analysis of the clinical responsiveness of the PNQ score and CTCAE assessment was limited to eight treatment cycles because the number of patients who continued over ten treatment cycles was limited. The worst values of sensory PNQ were 16 (31%), 20 (39%), 10 (20%), 4 (8%), and 1 (2%) for A, B, C, D, and E, respectively. The worst values of motor PNQ were 16 (31%), 16 (31%), 11 (22%), 4 (12%), and 2 (4%) for A, B, C, D, and E, respectively. The incidence of sensory or motor neuropathy in the PNQ score and CTCAE assessment increased as the number of treatment cycles increased (cycles 2, 4, 6, and 8: P<0.001, P=0.02, P<0.001, and P=0.004, respectively) (Figure 2).

We also examined the concordance between PNQ and CTCAE assessments. The reported PNQ scores were distributed over the full range (A to E), whereas none of the patients had grade 3 neurotoxicity on CTCAE assessment. In particular, three patients reported that their symptoms were of maximum severity (E) for motor disturbance using the PNQ score, whereas all physicians stated that these patients had no symptoms (0). The concordance between the PNQ score and CTCAE assessment for sensory and motor peripheral neuropathy showed low scores with kappa =0.49 and kappa = 0.10, respectively (Table 3).
Table 3
| Kappa =0.49 | CTCAE sensory | Kappa =0.10 | CTCAE motor | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | 0 | 1 | 2 | ≥3 | ||
| PNQ sensory | PNQ motor | ||||||||
| A | 90 | 1 | 1 | 0 | A | 84 | 2 | 0 | 0 |
| B | 20 | 41 | 6 | 0 | B | 49 | 5 | 0 | 0 |
| C | 2 | 13 | 7 | 0 | C | 15 | 5 | 2 | 0 |
| D | 2 | 0 | 4 | 0 | D | 3 | 2 | 3 | 0 |
| E | 0 | 0 | 0 | 0 | E | 3 | 0 | 0 | 0 |
PNQ, Patient Neurotoxicity Questionnaire; CTCAE, Common Terminology Criteria for Adverse Events.
Discussion
This prospective phase II study of weekly nab-paclitaxel for previously treated advanced NSCLC demonstrated moderate efficacy, with an ORR of 18.5% (95% CI: 10.9–29.6%), and a median PFS of 3.4 months. Safety profiles of weekly nab-paclitaxel were generally acceptable, with 30.8% patients experiencing severe grade neutropenia and 6.2% experiencing febrile neutropenia.
The number of treatment options for chemo-naïve patients with advanced NSCLC has increased due to the development of ICIs and tyrosine kinase inhibitors. However, most of these patients acquire resistance and require salvage treatment with cytotoxic chemotherapies. In this setting, DTX, PEM, and S-1 have been established as having clinical benefits for recurrent NSCLC. DTX demonstrated a survival benefit compared with best supportive care (BSC) in a randomized phase III study (20). PEM showed similar survival benefits with mild toxicity profiles compared with DTX (21). S-1 is an oral cytotoxic drug that comprises tegafur, gimeracil, and oteracil potassium. S-1 demonstrated non-inferiority in OS to DTX as second- or third-line therapy for patients with advanced NSCLC in a randomized, phase 3 study (24). However, ORRs of these agents are limited (5–9%), and new therapeutic options are needed.
Nab-paclitaxel monotherapy has been evaluated as an option in salvage settings for patients with recurrent NSCLC. Previous clinical trials of nab-paclitaxel monotherapy are listed in Table 4 (4-12). The efficacy of nab-paclitaxel for relapsed NSCLC has been investigated, and ORR and median PFS were found to be 7.3–31.7% and 2.0–5.1 months. The primary endpoint of this study was the ORR, assessed by the investigators. The lack of an independent central review is an important limitation in this study. However, we observed therapeutic effects similar to those reported previously for nab-paclitaxel monotherapy. The median PFS time was slightly shorter than that reported in previous clinical trials. This may be due to 36.9% of the enrolled patients being on the third-line chemotherapy. These results were confirmed in the J-AXEL study, which measured an ORR of 29.9% and a median PFS of 4.2 months. Based on these results, nab-paclitaxel monotherapy has become a treatment option for patients with relapsed NSCLC, as it has a higher response rate and manageable toxicity. In our clinical practice, a combination of DTX and ramucirumab has been the standard therapy with high response rate, longer PFS, and OS compared with the DTX monotherapy. However, there are some cases where it is difficult to ramucirumab due to the risk of bleeding or problematic comorbidities. In such cases, nab-paclitaxel may be a useful option as an alternate regimen with a high response rate and tolerability.
Table 4
| First author, year | Design | N | Dose | Line | ECOG PS | ORR (%) [95% CI] | PFS (months) [95% CI] | OS (months) [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Rizvi NA, 2008 (4) | Single-arm phase I/II | 60 (PI: n=12; PII: n=40) | 100 and 125 mg/m2; days 1, 8, 15; q4w | 1st | 70–100% (KPS) | 30 [16–44] | 5 [3–8] | 11 [7–NR] |
| Liu Z, 2015, (5) | Randomized phase II | 111 (PEM: 56 vs. nab-paclitaxel: 55) | 150 mg/m2; days 1, 8; q3w | 2nd | 0–2 | 14.5 [NE] | 5.1 [3.9–7.4] | 9.9 [8.2–11.9] |
| Hu W, 2015, (6) | Single-arm phase II | 56 | 100 mg/m2; days 1, 8, 15; q4w | 2nd | 0–2 | 16.1 [8.9–24.7] | 3.5 [1.9–5.8] | 6.8 [4.7–9.3] |
| Sakata S, 2016, (7) | Single-arm phase II | 41 | 100 mg/m2; days 1, 8, 15; q3w | 2nd | 0–2 | 31.7 [19.3–44.1] | 4.9 [2.4–7.4] | 13 [8.0–18.0] |
| Tanaka H, 2017, (8) | Single-arm phase II | 31 | 100 mg/m2; days 1, 8, 15; q4w | 2nd or further | 0–2 | 19.3 [9.1–36.2] | 4.5 [3.5–6.3] | 15.7 [11.7–NR] |
| Anzai M, 2017, (9) | Single-arm phase II | 32 | 100 mg/m2; days 1, 8, 15; q4w | 2nd | 0–2 | 28.1 [NE] | 3.9 [2.7–5.1] | 10.9 [9.5–12.3] |
| Wu Y, 2017, (10) | Randomized placebo-controlled phase II | 90 (placebo: 45 vs. nab-paclitaxel: 45) | 150 mg/m2; days 1, 8, 15; q4w | 2nd | 0–3 | 19.6 [NE] | 2.0 [0.9–4.3] | 4.9 [2.1–5.9] |
| Harada D, 2019, (11) | Single-arm phase I/II | 60 (PI: n=5; PII: n=55) | 100 mg/m2; days 1, 8, 15; q4w | 2nd or 3rd | 0–2 | 7.3 [2.0–17.6] | 3.4 [1.9–4.0] | 10.6 [6.9–17.8] |
| Yoneshima Y, 2021, (12) | Randomized phase III | 503 (DTX: 251 vs. nab-paclitaxel: 252) | 100 mg/m2; days 1, 8, 15; q3w | 2nd or 3rd | 0–1 | 29.9 [24.0–36.2] | 4.2 [3.9–5.0] | 16.2 [14.4–19.0] |
PI, phase I; PII, phase II; PEM, pemetrexed; nab-paclitaxel, nanoparticle albumin-bound paclitaxel; DTX, docetaxel; COG, Eastern Cooperative Oncology Group; PS, performance status; KPS, Karnofsky performance status; ORR, overall response rate; CI, confidence interval; NE, not evaluable; PFS, progression-free survival; OS, overall survival; NR, not reached.
Neurotoxicity remains a problematic side effect of paclitaxel-based therapy. We also investigated numbness using PNQ score as an additional measure and evaluated the effects of nab-paclitaxel on QOL. The frequency and severity of sensory and motor neuropathy increased significantly with each treatment cycle in both PNQ and CTCAE evaluations. The concordance rate between PNQ score and CTCAE for sensory and motor peripheral neuropathy showed low scores with kappa =0.49 and kappa =0.10, respectively. These results reveal an obvious gap between objective evaluation by patients and subjective evaluation by primary physicians, which demonstrates the difficulty of subjective evaluation of neurotoxicity due to nab-paclitaxel. This gap may lead to deficit of rest periods and appropriate treatment reduction in clinical settings. In particular, the concordance rate for motor peripheral neuropathy was low. This is because physicians usually enquire about sensory neuropathy as a closed question during physical examination. However, only a few physicians enquire about motor peripheral neuropathy. Currently, combination therapy with ICIs and cytotoxic chemotherapy is one of the standard treatments. Examples of this include atezolizumab plus bevacizumab plus CBDCA plus paclitaxel for adenocarcinoma (25), and CBDCA and either paclitaxel or nab-paclitaxel for squamous cell carcinoma (26). Taxane-based anticancer drugs will continue to be used frequently in the future, and this PRO analysis is expected to be useful for their treatment.
The present study has several limitations. First, in this study, patients with a history of ICI treatment were not included. Currently, immunotherapy is often used as a first-line treatment in anti-programmed cell death-1 (PD1) antibody monotherapy, which involves combination of immunotherapy and chemotherapy, as well as anti-PD1 and anti-cytotoxic T-lymphocyte antigen-4 (CLTA-4) antibodies. Therefore, patient background in this study may not resemble that of other patients with NSCLC. Little is known about nab-paclitaxel monotherapy after immunotherapy and further studies are needed. Second, a potential limitation of this study was the low collection rate of PNQ. One reason is that responding to the questionnaire required extra effort by both patients and physicians. Although this study shows the importance of PRO, confirming the results of previous studies (13,17,18), using PRO routinely in clinical settings may be difficult because of its complexity. However, as previously mentioned, evaluations of numbness made using CTCAE may be underestimates. Therefore, in the future, new assessment methods, such as using a mobile device, should be considered to assess numbness without extra effort. Third, because this study and other studies using nab-paclitaxel were mainly performed on Asian patients, these findings might not be directly generalizable to non-Asian patients. With regard to the effects of nab-paclitaxel in combination with CBDCA, it has been shown that the overall results were similar to those obtained for the Japanese subgroup in a global phase III study (3,14). This indicates that the results of nab-paclitaxel trials conducted on Asian subjects might be applicable to non-Asian populations.
In conclusion, the present study demonstrates that nab-paclitaxel monotherapy is well tolerated and has antitumor activity in patients with previously treated NSCLC. Therefore, nab-paclitaxel appears to be a treatment option for patients with previously treated NSCLC.
Acknowledgments
We would like to thank Editage (https://www.editage.com/) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-89/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-89/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-89/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-89/coif). SS reports receiving personal fees from AstraZeneca, Chugai Pharma, Taiho Pharmaceutical, and MSD outside of the submitted work. SM received lecture fees from Bristol-Myers, Ono Pharmaceutical, Boehringer Ingelheim, Eli Lilly, MSD, Chugai Pharma, AstraZeneca, Taiho Pharmaceutical, Kyowa Hakko Kirin, Pfizer, Novartis, Daiichi Sankyo, and Abbive outside of the submitted work. SW reports receiving personal fees from Eli Lilly, Pfizer, Novartis Pharma, AstraZeneca, Chugai Pharma, Bristol-Myers, Boehringer Ingelheim, MSD, Ono Pharmaceutical, Daiichi Sankyo, and Taiho Pharmaceutical outside of the submitted work. AO reports receiving personal fees from Daiichi Sankyo and Nipro Corporation outside of the submitted work. KN reports receiving personal fees from Bristol-Myers, Pfizer, and Novartis outside of the submitted work. KI reports receiving personal fees from AstraZeneca, Chugai Pharma, Bristol-Myers, Boehringer Ingelheim, Ono Pharmaceutical, Taiho Pharmaceutical, Novartis, and Daiichi Sankyo. KK reports receiving personal fees from Ono Pharmaceutical, Chugai Pharmaceutical and AstraZeneca outside of the submitted work. HT reports receiving personal fees from Chugai Pharmaceutical and AstraZeneca outside of the submitted work. TA reports receiving personal fees from AstraZeneca, Chugai Pharma, Boehringer Ingelheim, Eli Lilly, Taiho Pharmaceutical, Viatris, ONO Pharmaceutical, Novartis, Nipponkayaku, and Bristol-Myers outside of the submitted work. TO reports receiving personal fees from AstraZeneca, Chugai Pharma, Bristol-Myers, Taiho Pharmaceutical, and Kyowa Hakko Kirin outside of the submitted work. KS reports receiving personal fees from AstraZeneca, Ono Pharmaceutical, Novartis Pharma, GSK, and Berlinger Japan. HY reports receiving personal fees from AstraZeneca, Boehringer Ingelheim, Taiho Pharmaceutical, Chugai Pharma, and Ono Pharmaceutical outside of the submitted work. TK reports receiving personal fees and grant from Chugai Pharma, Boehringer Ingelheim, Eli Lilly, MSD, Taiho Pharmaceutical, Daiichi Sankyo, Ono Pharmaceutical AstraZeneca, Shionogi, TEIJIN PHARMA, and KYORIN Pharmaceutical, and lecture fees from Astellas Pharma, Bristol-Myers, Pfizer, Taisho Toyama Pharmaceutical, Janssen Pharmaceutical, Japan BCG Laboratory Novartis, Mylan N.V., and Roche Diagnostics outside of the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. The study was approved by the Ethics Committee of Niigata University (No. NH25-006) and informed consent was taken from all the patients. The study was registered with the University Hospital Medical Information Network (UMIN) with the clinical trial number UMIN000012343.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Green MR, Manikhas GM, Orlov S, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol 2006;17:1263-8. [Crossref] [PubMed]
- Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 2006;12:1317-24. [Crossref] [PubMed]
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [Crossref] [PubMed]
- Rizvi NA, Riely GJ, Azzoli CG, et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol 2008;26:639-43. [Crossref] [PubMed]
- Liu Z, Wei Z, Hu Y, et al. A phase II open-label clinical study of comparing nab-paclitaxel with pemetrexed as second-line chemotherapy for patients with stage IIIB/IV non-small-cell lung cancer. Med Oncol 2015;32:216. [Crossref] [PubMed]
- Hu W, Zhang Z. A phase II clinical study of using nab-paclitaxel as second-line chemotherapy for Chinese patients with advanced non-small cell lung cancer. Med Oncol 2015;32:498. [Crossref] [PubMed]
- Sakata S, Saeki S, Okamoto I, et al. Phase II trial of weekly nab-paclitaxel for previously treated advanced non-small cell lung cancer: Kumamoto thoracic oncology study group (KTOSG) trial 1301. Lung Cancer 2016;99:41-5. [Crossref] [PubMed]
- Tanaka H, Taima K, Morimoto T, et al. A single-arm phase II study of nab-paclitaxel for patients with chemorefractory non-small cell lung cancer. BMC Cancer 2017;17:683. [Crossref] [PubMed]
- Anzai M, Morikawa M, Okuno T, et al. Efficacy and safety of nanoparticle albumin-bound paclitaxel monotherapy as second-line therapy of cytotoxic anticancer drugs in patients with advanced non-small cell lung cancer. Medicine (Baltimore) 2017;96:e9320. [Crossref] [PubMed]
- Wu Y, Feng J, Hu W, et al. A randomized placebo-controlled clinical study of nab-paclitaxel as second-line chemotherapy for patients with advanced non-small cell lung cancer in China. Biosci Rep 2017;37:BSR20170020. [Crossref] [PubMed]
- Harada D, Kozuki T, Nogami N, et al. A phase I/II trial of weekly nab-paclitaxel for pretreated non-small-cell lung cancer patients without epidermal growth factor receptor mutations and anaplastic lymphoma kinase rearrangement. Asia Pac J Clin Oncol 2019;15:250-6. [Crossref] [PubMed]
- Yoneshima Y, Morita S, Ando M, et al. Phase 3 Trial Comparing Nanoparticle Albumin-Bound Paclitaxel With Docetaxel for Previously Treated Advanced NSCLC. J Thorac Oncol 2021;16:1523-32. [Crossref] [PubMed]
- Shimozuma K, Ohashi Y, Takeuchi A, et al. Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer 2009;17:1483-91. [Crossref] [PubMed]
- Satouchi M, Okamoto I, Sakai H, et al. Efficacy and safety of weekly nab-paclitaxel plus carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer 2013;81:97-101. [Crossref] [PubMed]
- National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer 2016;24:3669-76. [Crossref] [PubMed]
- Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 2006;7:903-9. [Crossref] [PubMed]
- Kuroi K, Shimozuma K, Ohashi Y, et al. Prospective assessment of chemotherapy-induced peripheral neuropathy due to weekly paclitaxel in patients with advanced or metastatic breast cancer (CSP-HOR 02 study). Support Care Cancer 2009;17:1071-80. [Crossref] [PubMed]
- Hausheer FH, Schilsky RL, Bain S, et al. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 2006;33:15-49. [Crossref] [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. [Crossref] [PubMed]
- Nokihara H, Lu S, Mok TSK, et al. Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer). Ann Oncol 2017;28:2698-706. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]


