
Prospective evaluation of immunological, molecular-genetic, image-based and microbial analyses to characterize tumor response and control in patients with unresectable stage III NSCLC treated with concurrent chemoradiotherapy followed by consolidation therapy with durvalumab (PRECISION): protocol for a prospective longitudinal biomarker study
Introduction
In PD-L1 positive (≥1%), unresectable stage III non-small cell lung cancer (NSCLC), concurrent platinum-based chemoradiotherapy (CRT) followed by maintenance treatment with the PD-L1 inhibitor durvalumab represents the new standard of care with approval of the European Medicines Agency (EMA) based on the promising results of the PACIFIC study (1-6). However, prognostic and predictive markers for this novel tri-modal treatment approach remain unclear. Historically, aspects such as patients’ general condition, pulmonary function as well as tumor volume, extent of the tumor invasion and lymph node involvement have been of crucial importance for prognosis and treatment efficacy (7-9). Due to the introduction of immune check-point inhibition in contemporary cancer treatment, the state of the host’s immunity and microbiome has become more relevant for treatment efficacy and prognosis (10-12).
In order to identify a framework of prognostic and predictive biomarkers in unresectable stage III NSCLC, we will perform a non-interventional explorative biomarker study with longitudinal comprehensive characterization of the patient (host) and tumor as well as their longitudinal changes during the course of tri-modal therapy. This study will provide invaluable information on the immunological, molecular-genetic, morphological and microbial parameters in patients with unresectable stage III NSCLC treated with durvalumab maintenance for 12 months after concurrent CRT. The following article was written in accordance with the SPIRIT reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-1010/rc).
Methods
The study protocol and informed consent documents were approved by the ethics committee of the Ludwig-Maximilians-University of Munich (approval number: 20-0502), and informed consent will be obtained from all patients. All procedures performed in this study will be in accordance with the Declaration of Helsinki (as revised in 2013) and adhere to Good Clinical Practice guidelines.
Study design and patients
This non-interventional hypothesis-generating single-center biomarker study aims at a longitudinal comprehensive phenotyping of the patient (host) and the tumor regarding molecular-genetic features, immunological, morphological, image-based and microbial characteristics with additional assessment of changes during tri-modal treatment and potentially recurrent disease. We will enroll 40 patients with unresectable stage III NSCLC who will receive concurrent platinum-based CRT followed by maintenance therapy with the PD-L1 inhibitor durvalumab at the Department of Radiation Oncology, University Hospital Munich (Ludwig-Maximilians-University; LMU).
All patients will be discussed at the thoracic oncology tumor board of the University Hospital Munich (LMU) and will be screened for eligibility in the study regarding histopathological and radiological findings and patients’ characteristics.
Key inclusion and exclusion criteria
- Inclusion criteria:
- Patient age ≥18 years;
- Histologically/cytologically confirmed diagnosis of NSCLC;
- Patients with unresectable NSCLC and tumor stage III A–C according to the Union Internationale Contre le Cancer (UICC) TNM 8th edition;
- Eligible for concurrent platinum-based CRT followed by durvalumab maintenance treatment according to the latest approval in Germany;
- No invasive malignancy (mesenchymal, hematological or epithelial) in the five preceding years;
- Eastern Cooperative Oncology Group (ECOG) performance status 0–2;
- Lung function parameters (before or after bronchodilation): FEV1 ≥1.0 L and/or DLCO-SB ≥40% predicted;
- A maximum of two cycles of induction chemotherapy are permissible before the start of CRT.
- Exclusion criteria:
- Simultaneous participation in another clinical trial;
- Mixed histology of small-cell lung cancer and NSCLC;
- Brain metastases confirmed by a contrast enhanced cranial MRI;
- Prior receipt of immunotherapy or an investigational medicinal product;
- Previous exposure to an anti-PD-1 or anti-PD-L1 antibody;
- Pneumonitis ≥ grade 2 as a result of prior CRT;
- Patients with a non-active disease in the last 5 years can be included, but only after consultation with the responsible investigator of the study or his representative;
- Primary immunodeficiencies in previous history
- Prior interstitial lung disease (ILD);
- Prior autoimmune disease;
- Previous organ transplantation with subsequent therapeutic immunosuppression.
After consent and enrollment, blood samples will be collected during clinical routine 5–10 days before the start of CRT and subjected to immunophenotyping of peripheral blood mono-nuclear cells (PBMC), to genetic testing using the Oncomine Pan-Cancer Cell free Assay (Life Technologies, Carlsbad, CA, USA) next generation sequencing (NGS) as well as the detection of mutations in short circulating tumor total nucleic acid (ctTNA: ctDNA and ctRNA). At the same time of blood sampling, saliva and stool sample for microbiome analyses will be collected.
During treatment, a comprehensive characterization including immunophenotyping of PBMC, ctDNA as well as gut/saliva microbiome analyses will be performed.
Immunophenotyping will be performed using a set of eligible markers (CD3, CD4, CD8, CD19, CD20, CD14, CD16, slan, HLA-DR) to quantify the major blood cell lineages like T-cells, B-cells, NK-cells, and monocytes including their subpopulations in absolute numbers and frequencies. Further marker panels (e.g., FoxP3, Ki67, CD25, CX3CR1, CD38) are used to assess immunocyte subpopulations like Tnaive, Treg, Teff, Tmem, Texh that are relevant in immune response, regulation, and killing.
Bacterial assemblages will be analyzed using high-throughput sequencing based on 16S rRNA profiles to selectively identify the transcriptionally active bacteria. RNA from saliva and faecal samples will be extracted using the RNeasy kit (Qiagen, Mississauga, ON, Canada). After DNA digestion, first-strand cDNA will be synthesized using Superscript IV First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) and random primers. Amplicon libraries will be generated as described before in detail (13,14).
Sample collection will be repeated mid-treatment (at the 15 fraction mark), at the end of CRT and 3, 6 and 12 months after the end of thoracic irradiation (see Figure 1).
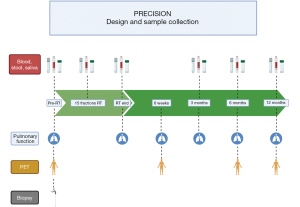
18F-FDG-PET/computed tomography (CT) will be performed 5–10 days before start of radiotherapy (RT), 6 weeks, 6, 12 and 24 months after the end of CRT. Patients with asymptomatic disease progression will be assessed according to our internal standard operating procedure (SOP) with CT of the chest and abdomen every 3 months and every 6 months an MRI of the brain. In patients with symptomatic disease, imaging modality will depend on physician’s choice followed by the internal SOP.
Lung function will be assessed before the start of, at the end and 6 weeks as well as 3, 6 and 12, 18, 24 months after CRT.
Histopathological examination and NGS will be performed at the accredited Institute of Pathology (Faculty of Medicine, LMU of Munich, Munich, Germany).
Follow-up until 5 years after the end of chemoradiation will be performed by the Department of Radiation Oncology at the University Hospital Munich (LMU) according to the clinical SOPs as well as treatment-related side effects according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.
Study objectives
The primary objective is to identify early immunological and morphological biomarkers and their dynamic changes to predict progression-free survival (PFS) at 12 and 24 months [according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, and PERCIST as assessed by means of blinded independent central review]. PFS is defined as the time from end of CRT to the date of the first documented event of tumor progression or death in the absence of disease progression.
The secondary objective is to identify predictive biomarkers for PFS at 6 and 18 months after CRT. PFS will be assessed by the investigators according to RECIST 1.1 and PERCIST. Overall response rate, local and distant tumor control (response duration) at 6 weeks, 6, 12, 18 and 24 months from the end of CRT according to PERCIST and RECIST criteria will be correlated with potential biomarkers.
Statistical analysis
In this prospective explorative study, 40 patients will be enrolled between December 2020 and September 2022 based on previous single-center experience and published historical data:
- 20 patients will be progression-free at 24 months after the start of CRT (50% PFS rate);
- 12 patients will develop multiple distant metastases, including brain (30% rate);
- 3 patients will develop a single metachronous distant metastasis, including brain (7.5% rate);
- 10 patients will develop a loco-regional recurrence (25% rate) at 24 months after completion of CRT.
Five cancer-related deaths are anticipated until end of follow-up period (24 months after start of CRT). A 5% drop-out rate was estimated.
With a sample size of 40 patients, an explorative biomarker study using multiple testing is justified to evaluate predictive and prognostic biomarker by the scarcity of quality samples, capacity and costs of molecular techniques. A further confirmation step is planned in an external validation cohort.
Discussion
Implementation of maintenance treatment with the PD-L1 inhibitor durvalumab in unresectable stage III NSCLC with at least stable disease after completion of concurrent CRT has led to a marked improvement in patient prognosis and established a new standard approach for this heterogeneous disease. According to the PACIFIC trial and reported real-world data, this novel tri-modal approach has led to a significant increase in local-regional control, PFS and overall survival (OS) (5,14).
Despite the unprecedented historical improvement of long-term outcome, a significant number of patients will develop recurrent disease. Importantly, there is a paucity of data regarding salvage treatment options. Comprehensive analysis of recurrence predictors, including clinical factors and biomarker candidates are pertinent.
Additionally, further intensification of this tri-modal approach with application of immune checkpoint inhibition and CRT simultaneously followed by the established maintenance treatment is also the subject of several ongoing studies (15,16).
In the face of the achieved progress in this field, underlying mechanisms explaining the durable positive effect of durvalumab maintenance treatment on local and distant tumor control after CRT are still unclear (3,17,18). Hence, the ongoing trials should be focused on deciphering these synergistic effects.
PRECISION is a single-center explorative biomarker study designed to generate a framework of specific tumor- and patient (host)-associated parameters with significant impact on the processes of tumor clearance during CRT and establishment of long-lasting antitumor immune response during the durvalumab maintenance phase of multimodal treatment.
The strength of this study is a comprehensive and longitudinal biological material collection and analysis. Analyses will be performed at six time points: three time points during CRT and three during the maintenance treatment phase, respectively. This study will also evaluate the dynamic changes of the tumor- and patient (host)-related parameters across the treatment time. The association of the detected cfDNA/ctDNA levels/variant frequencies with objective tumor response based on the RECIST and PERCIST criteria and pattern of failure (loco-regional vs. distant) may add new relevant data for patient follow-up. Correlation of the dynamic changes of PBMCs with acute and subacute treatment-related toxicity, especially pneumonitis, should provide crucial knowledge for future studies to mitigate adverse effects.
Previous studies found a mutual relationship between the gut microbiota and administered immunotherapy especially in malignant melanoma patients (19,20). Despite an unknown underlying mechanism, gut microbes may modulate adaptive immunity, either in a beneficial or harmful manner and might influence the efficacy and toxicity of immunotherapy via the microbiota and modulation of adaptive immunity. Our study contributes to a further understanding of the patient immune system and identify potential biomarkers. In order to confirm our results, a validation preferably in an external cohort is warranted and planned.
In summary, PRECISION will provide invaluable information regarding patient (host) systemic immune landscape and the cancer—patient (host) immune interplay at different phases of tri-modal treatment. The key challenge will be defining a specific framework of immunological, molecular-genetic and microbial parameters and their dynamic changes with specific impact on long-term tumor control and patient prognosis.
Acknowledgments
Figure 1 of this article has been reproduced from Käsmann et al. (15) and written permission from the copyright holder (Springer Nature) has been obtained in order to reproduce the material.
Funding: The Hospital of the LMU of Munich takes the sponsor role for this study. The study was supported by an unrestricted grant from AstraZeneca Germany.
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-1010/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-1010/coif). AJ receives honoraria, worked in consulting or advisory role and received expenses for travel and accomodations by Amgen, Astra Zeneca, Bayer Pharmaceuticals, BMS, Boehringer Ingelheim, Merck KGaA, Novartis, Qiagen, Roche Pharma and Takeda. AT serves in the advisory boards: Lilly, Pfizer, MSD, BMS, GSK, Celgene, Roche, Takeda, Boehringer Ingelheim, Amgen, AstraZeneca. Projects financed by AstraZeneca und Takeda. AT receives travel support from Pfizer and Amgen. CB receives grants or contracts from any entity but not related to this manuscript from Viewray, Brainlab and ELEKTA. CB receives honoraria from BMS, ROCHE, MERCK, Astrazeneca and Viewray. CB receives support for attending meetings and/or travel from BMS, ROCHE, MERCK, Astrazeneca and Viewray. CB receives BMS, ROCHE, MERCK, Astrazeneca and Viewray. CB serves in a fiduciary role in the ESTRO. CE receives grants or contracts from any entity but not related to this manuscript from German Cancer Aid. CE receives consulting fees from Novartis. CE receives support for attending meetings and/or travel from Novartis. CS has received lecture honoraria from Falk and Janssen. EN receives honoraria from Astrazeneca, Hexal, Ipsen and Roche. EN serves in consulting or advisory board of Definiens and Visiopharm. EN receives research funding from Phio Pharmaceuticals (Inst). EN receives royalties or licenses from Medigne (Inst). FK is co-founder of the company ‘Aignostics GmbH’ and receives honoraria from BMS, Merck, Agilent, Novartis, Roche, Lilly, MSD. FM receives an unrestricted Research Institutional Grant from AstraZeneca. FM receives honoraria from AstraZeneca, Novartis, Roche, Lilly, Elekta and Brainlab. FM serves in the advisory board of AstraZeneca, Novartis. LK receives honoraria from AMGEN. NR reports receiving honoraria for speaker activities and participation in advisory boards for AbbVie, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, Roche, and Takeda. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This biomarker study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013) and adhere to Good Clinical Practice guidelines. Prior to the initiation of the study, local Ethics Committee/Institutional review board approval (approval number: 20-0502) has been given, and informed consent will be obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Käsmann L, Eze C, Taugner J, et al. Chemoradioimmunotherapy of inoperable stage III non-small cell lung cancer: immunological rationale and current clinical trials establishing a novel multimodal strategy. Radiat Oncol 2020;15:167. [Crossref] [PubMed]
- Taugner J, Käsmann L, Karin M, et al. Planning target volume as a predictor of disease progression in inoperable stage III non-small cell lung cancer patients treated with chemoradiotherapy and concurrent and/or sequential immune checkpoint inhibition. Invest New Drugs 2022;40:163-71. [Crossref] [PubMed]
- Taugner J, Käsmann L, Eze C, et al. Durvalumab after Chemoradiotherapy for PD-L1 Expressing Inoperable Stage III NSCLC Leads to Significant Improvement of Local-Regional Control and Overall Survival in the Real-World Setting. Cancers (Basel) 2021;13:1613. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. [Crossref] [PubMed]
- Käsmann L, Niyazi M, Blanck O, et al. Predictive and prognostic value of tumor volume and its changes during radical radiotherapy of stage III non-small cell lung cancer: A systematic review. Strahlenther Onkol 2018;194:79-90. [Crossref] [PubMed]
- Ostheimer C, Mäurer M, Ebert N, et al. Prognostic impact of gross tumor volume during radical radiochemotherapy of locally advanced non-small cell lung cancer-results from the NCT03055715 multicenter cohort study of the Young DEGRO Trial Group. Strahlenther Onkol 2021;197:385-95. Erratum in: Strahlenther Onkol 2021;197:560-1. [Crossref] [PubMed]
- Taugner J, Käsmann L, Eze C, et al. Survival score to characterize prognosis in inoperable stage III NSCLC after chemoradiotherapy. Transl Lung Cancer Res 2019;8:593-604. [Crossref] [PubMed]
- Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91-7. [Crossref] [PubMed]
- Hakozaki T, Richard C, Elkrief A, et al. The Gut Microbiome Associates with Immune Checkpoint Inhibition Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Immunol Res 2020;8:1243-50. [Crossref] [PubMed]
- Hatae R, Chamoto K, Kim YH, et al. Combination of host immune metabolic biomarkers for the PD-1 blockade cancer immunotherapy. JCI Insight 2020;5:133501. [Crossref] [PubMed]
- Frost F, Kacprowski T, Rühlemann MC, et al. Functional abdominal pain and discomfort (IBS) is not associated with faecal microbiota composition in the general population. Gut 2019;68:1131-3. [Crossref] [PubMed]
- Frost F, Kacprowski T, Rühlemann M, et al. Long-term instability of the intestinal microbiome is associated with metabolic liver disease, low microbiota diversity, diabetes mellitus and impaired exocrine pancreatic function. Gut 2021;70:522-30. [Crossref] [PubMed]
- Käsmann L, Manapov F. PRECISION: Radiochemotherapie gefolgt von Durvalumab-Erhaltungstherapie im inoperablen Stadium III NSCLC – eine prospektive Biomarkerstudie. Forum 2021. Available online:
10.1007/s12312-021-00993-2 10.1007/s12312-021-00993-2 - Antonia S, Villegas A, Daniel D, et al. Overall survival with durvalumab versus placebo after chemoradiotherapy in stage III NSCLC: updated results from PACIFIC. J Thorac Oncol 2018;13:S184. [Crossref]
- Landman Y, Jacobi O, Kurman N, et al. Durvalumab after concurrent chemotherapy and high-dose radiotherapy for locally advanced non-small cell lung cancer. Oncoimmunology 2021;10:1959979. [Crossref] [PubMed]
- Käsmann L, Eze C, Taugner J, et al. Implementation of durvalumab maintenance treatment after concurrent chemoradiotherapy in inoperable stage III non-small cell lung cancer (NSCLC)-a German radiation oncology survey. Transl Lung Cancer Res 2020;9:288-93. [Crossref] [PubMed]
- Zhou CB, Zhou YL, Fang JY. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 2021;7:647-60. [Crossref] [PubMed]
- Lee KA, Thomas AM, Bolte LA, et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med 2022;28:535-44. [Crossref] [PubMed]


