
The procedure and effectiveness of release maneuvers in tracheobronchial resection and reconstruction
Introduction
Only 2% of airway malignancies are located in the trachea, making it a rare disease (1-3). Resection and reconstruction is a radical surgical treatment for tracheal malignancy (4,5). Improved understanding of the procedure and anatomic structures involved can facilitate a low-tension, well-perfused anastomosis, leading to better patient outcomes (3). Previously, the length of tracheal resection was limited to 2 cm, which was proposed by Rob and Belsey in 1948. They reported a series of cases and suggested 2 cm should be the extent for primary tracheal resection and reconstruction (6,7). The lack of understanding of tracheal blood supply limits resection length and surgeons were unable to mobilized the trachea extensively, hindering tension-free anastomosis.
Subsequently, blood supply mapping studies of the trachea describing the segmental characteristics were reported (8,9). With the help of these studies, Broussard and Mathisen reported that neck flexion and paratracheal dissection allowed 4 cm of tracheal resection without anastomotic complication (9). However, patients with longer tracheal lesions faced either limited treatment options or anastomotic complications following aggressive surgery. Therefore, additional release maneuvers were performed and reported to be effective to extend the tracheal resection boundary to 6 cm (1,9,10). A series of tracheal release maneuvers were previously reported by Broussard and Mathisen including suprahyoid, paracardial release maneuvers. However, seldomly studies had reported surgical outcomes or postoperative complications after the release maneuvers (9). The current study aimed to report on cases of complicated tracheal tumor patients who had undergone tracheal resection and reconstruction with additional release maneuvers. The release procedures performed and the postoperative outcomes are described. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-385/rc).
Methods
Patients
It is a case series study that the patients with tracheal or bronchial lesions who underwent tracheobronchial resection and airway reconstruction at the First Affiliated Hospital of Guangzhou Medical University from January 1st 2019 to December 31st 2021 are identified. The patients’ clinical characteristics were recorded, including their age, sex, tumor location, release maneuver, pathology results, and complications. In addition to tracheobronchial neoplasms (n=45), benign stenotic cases (n=22) were also considered in order to maximize the primary cohort. Those who received sleeve resection without release maneuver were excluded from the study. One case of tracheal tumor involving carina and left main bronchus received veno-arterial extracorporeal membrane oxygenation (V-A ECMO) tracheobronchial resection and reconstruction for the sake of extensive release maneuver and stable hemodynamics. The surgical outcomes including the postoperative intensive care unit (ICU) stay, hospital stay, complications and vital status were recorded during the follow-up process. The efficacy and safety of the release maneuvers would be analyzed.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2020K-43). The individual consent for the retrospective study was signed and collected from the patients or their legal guardians. The data and the medical images would be showed with their official permission.
Preoperative evaluation
The preoperative evaluation and planning of patients receiving tracheal resection and reconstruction is of paramount importance. Patient history, physical exam, radiology test, and bronchoscopy results were included. Laryngoscopy for vocal cord examination and voice function is encouraged in all patients (11). Contrast computed tomography (CT) with 3-dimensional tracheobronchial reconstruction, including neck and chest, provided accurate lesion localization, showing the neck and mediastinal vascularized tissues. Patients with lesions in the cervical trachea or tracheal thoracic junction received a lateral cervical X-ray to characterize the position of the hyoid, thyroid, and cricoid bones. Based on the exam results, the resection length was estimated, and the release maneuver was discussed and planned. Patients with long resection length would be planned for release maneuver before operation.
Anesthesia
Non-intubated intravenous anesthesia would be applied in the suitable patients to facilitate the reconstruction surgery. Propofol target-controlled infusion mode with concentration of 1.5 to 3.0 µg/mL and midazolam 0.05 to 0.1 mg/kg were administered intravenously for sedation. Sufentanil (5 to 10 µg) was applied for analgesia during the induction process. The patient was maintained using propofol in target-controlled infusion mode (1.0 to 3.0 µg/mL) and remifentanil (0.03 to 0.05 µg/kg/min). Dexmedetomidine (0.5 to 1.0 µg/kg/h) would be also apply on demand. The laryngeal mask was used for oxygen delivery at a rate of 3.5 mL/min. Spontaneous respiration was maintained throughout the surgery. If the severe hypoxia occurred, an oxygen tube or high-jet ventilation tube would be inserted to the distal airway crossing the surgical field. The devices for tracheal intubation, video-assisted thoracic surgery (VATS) endobronchial intubation, and mechanical ventilation were prepared to prevent emergency situation (e.g., airway spasm or obstruction) and ensure the surgical safety.
Surgical technique
Incisions
A transverse cervical incision (collar incision) was performed for cervical lesions and invasive thyroid carcinoma invasion cases. A half sternotomy was also performed for lesions located at the junction of the cervical and thoracic trachea. A sternotomy incision was selected for thoracic and carinal lesions, especially in cases of main bronchus invasion. A lateral thoracotomy was applied in cases with bronchial lesions. In some thoracic and carinal cases without bronchial invasion, right thoracotomy for video-assisted thoracoscopy surgery was selected.
Laryngeal suprahyoid release
Laryngeal and suprahyoid release maneuvers have been reported to be beneficial for the extended resection of upper and middle tracheal lesions. However, routine use of these maneuvers is not encouraged as there are potential risks. Dedo et al. first reported laryngeal release between the thyroid cartilage and thyrohyoid muscle/membrane (12). It was not widely used because of the risk of laryngeal dysfunction. Montgomery developed the suprahyoid release maneuver in 1974, decreasing such risk (13). This method is generally accepted and used in various medical institutes, including Massachusetts General Hospital (9).
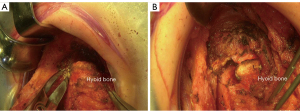
If the suprahyoid release is required, the cervical subfascial plane is dissected from the thyroid to the hyoid bone. The cephalad surface of the hyoid bone is then exposed and the bilateral digastric sling carefully preserved. The muscles between each side of the digastric sling are divided with cautery, including the mylohyoid, geniohyoid, and genioglossus muscles, and the tendons of the chondroglossus muscle (4,9). The hyoid bone is then divided on each side medial to the digastric sling with mayo scissors (Figure 1). A small drain is placed in the preepiglottic space, and the space is closed in 2 layers. Careful hemostasis is achieved because this area becomes inaccessible once tracheal anastomosis is performed and the neck is in flexion position.
Pericardial and hilar release
The pericardial and hilar release maneuver effectively allows for lower tracheal, carinal, and main bronchial resections. In the right thoracotomy approach, the right hilar, pericardial, and right inferior pulmonary ligament are dissected using cautery or harmonic scalpel. These release maneuvers are performed bilaterally in the median sternotomy approach, providing a better releasing effect. The pericardium is incised in a “U” shape posterior to the plane of the phrenic nerve. The surrounding tissue attaching the pericardium to the epicardium are divided. The superior and inferior pulmonary veins are dissected using cautery. Attention is paid to preserving the phrenic nerve and the main bronchial blood supply (Figure 2A,2B).
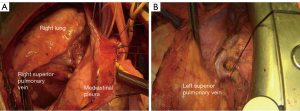
After opening the mediastinal pleura and pericardium, the thoracic trachea and the great vessels, including the brachiocephalic artery, the brachiocephalic vein, the superior vena cava, and the aorta, are thoroughly dissected and suspended with a rubber band (Figure 2A,2B).
Anastomosis
The proximal and distal resection margins are determined by intraoperative bronchoscopy. After resection is performed, the proximal and distal stumps are trimmed for anastomosis with a scalpel and scissors after the tracheal lesion resection. The intra-operative frozen section is performed to verify the margin clearance. The anesthesia approach is customized according to the patient’s condition. The non-intubated spontaneous respiratory anesthesia, which could facilitate and simplify the reconstruction process is applied to those who are tolerable. The cross-field intubation is prepared in case of insufficient ventilation. The patients are repositioned with their necks flexing to facilitate tension-free anastomosis. Prolene 2-0 sutures are placed on the lateral wall of each stump and are used as traction sutures. The membranous wall is anastomosed first with interrupted Vicryl 4-0 sutures. After the sutures are tied down, the cartilaginous wall of the trachea is then reconstructed using interrupted Vicryl 3-0 or 4-0 sutures. Since the blood supply from the peritracheal lymph node and tissue is crucial for anastomosis, the mediastinal lymph node sampling would be performed rather than complete dissection.
An air leak test with 30 cmH2O pressure is applied after reconstruction. An intra-operative bronchoscopy is performed to observe the anastomosis and sutures. The strap muscles are routinely used to buttress the cervical tracheal anastomosis. In thyroid carcinoma patients and pre-operative patients, the strap muscles are usually severely adherent and fibrosed, thus the sternocleidomastoid muscle can be used for buttressing instead. Pericardium and thymic tissue are used to buttress thoracic or carinal tracheal anastomosis. Patients are maintained in a semi-recumbent position with their necks remaining in flexion position for the early post-operative period. The Grillo neck stitches between pre-sternal and submental fascia would be applied to the cervical and cervico-thoracic cases. They would be removed 2 weeks after operation. But they are seldomly applied in the thoracic cases unless long resection length with intense-tension anastomosis. Prolonged antibiotics and prolonged hospital stay are defined as antibiotic use longer than 5 days and postoperative hospital stay longer than 10 days. The anastomotic is inspected via postoperative bronchoscopy. Anastomotic necrosis was defined as anastomosis covered by white and yellowish purulent tissue.
Statistical analysis
The number and clinical data of the included patients would be summarized. The continuous variables including age, height and weight etc. would be analyzed and demonstrated as mean ± standard deviation (SD). Meanwhile, the categorical variables would be depicted in number (No.) and the proportion of the total cohort (percentage, %). The statistical analyses were performed using SPSS 25 (IBM, USA).
Results
A total of 67 patients who had received release maneuvers for tracheobronchial surgeries were included. Males accounted for a larger proportion of the cohort (46/67, 65.7%). The mean age was 44.4 years, and the mean body mass index (BMI) was 22. The majority of patients had thoracic lesions (21/67, 31.4%). In addition, there were 17 (25.4%) patients with cervical lesions, 11 (16.4%) with junctional lesions, and 9 cases each of carinal (13.4%) and bronchial (13.4%) tumors. Among the cases, 2 patients had thyroid carcinoma with tracheal involvement and received laryngeal suprahyoid release. One patient was diagnosed with tumor recurrence 5 years after thyroid carcinoma resection. The majority of patients (39/67, 58.2%) underwent inferior pulmonary ligament release maneuvers with thoracic, carinal, and bronchial lesions. Those with cervical and junctional lesions received pretracheal release maneuvers (29/67, 43.3%). The hilar and pericardial release maneuvers was used in 10 patients (Table 1). Most of the patients received non-intubation anesthesia (51/67, 76.1%). VATS and robot-assisted thoracic surgery (RATS) were performed in 26 (41.8%) and 4 (6%) patients, respectively. The mean resection length is 3.26 cm (Table 1). Until the data and information were collected in the current study, the follow-up of the included patients was still in process.
Table 1
| Features | No./mean ± SD | Proportion (%)/median [range] |
|---|---|---|
| Sex | ||
| Male | 46 | 65.7 |
| Female | 21 | 34.2 |
| Age (years) | 44.4±16.3 | 43 [4–72] |
| Height (cm) | 164.6±11.0 | 165 [109–190] |
| Weight (kg) | 62.8±12.8 | 61 [15–94] |
| BMI (kg/m2) | 22.0±3.0 | 23 [13–29] |
| Lesion location | ||
| Cervical | 17 | 25.4 |
| Cervico-thoracic | 11 | 16.4 |
| Thoracic | 21 | 31.4 |
| Carinal | 9 | 13.4 |
| Bronchial | 9 | 13.4 |
| Release maneuver | ||
| Laryngeal suprahyoid | 2 | 3 |
| Pretracheal | 29 | 43.3 |
| Hilar/pericardial | 10 | 14.9 |
| Inferior pulmonary ligament | 39 | 58.2 |
| Anesthesia | ||
| Intubation | 16 | 23.9 |
| Non-intubation | 51 | 76.1 |
| Surgery | ||
| VATS | 26 | 41.8 |
| RATS | 4 | 6.0 |
| Open | 37 | 52.2 |
| Resection length (cm) | 3.26±1.12 | 3.5 [1.2–4.6] |
| Pathology | ||
| Squamous cell carcinoma | 10 | 14.9 |
| Adenoid cystic carcinoma | 18 | 26.9 |
| Mucoepidermoid carcinoma | 7 | 10.4 |
| Thyroid carcinoma | 2 | 3 |
| Other malignancy | 8 | 11.9 |
| Benign | 22 | 32.8 |
| ICU stay (hours) | 71.31±47.62 | 59 [28–245] |
| Hospital stay (days) | 9.17±6.17 | 7 [4–38] |
| Complications | ||
| Hoarseness | 6 | 9 |
| Prolonged antibiotics | 24 | 35.8 |
| Prolonged hospital stay | 18 | 26.9 |
| Anastomotic dehiscence | 0 | 0 |
| Anastomotic necrosis | 6 | 9 |
| Aspiration | 2 | 3 |
SD, standard deviation; BMI, body mass index; VATS, video-assisted thoracic surgery; RATS, robot-assisted thoracic surgery; ICU, intensive care unit.
Twenty-two patients were confirmed as having benign disease, including stenosis, bronchial cyst, glomus tumor, leiomyoma, schwannoma, and neurofibroma. The most commonly seen malignancies were adenoid cystic carcinoma (18/67, 26.9%), squamous cell carcinoma (10/67, 14.9%), and mucoepidermoid carcinoma (7/67, 10.4%). Other malignancies included thyroid carcinoma (4 cases), epithelial-myoepithelial carcinoma (EMC, 1 case), inflammatory myofibroblastic tumor (IMT, 1 case), lymphoepithelioma-like carcinoma (LELC, 1 case), and non-Hodgkin lymphoma (NHL, 2 cases). The median ICU stay was 59 hours, ranging from 28 to 245 hours, while the median hospital stay was 7 days, ranging from 4 to 38 days. There were 24 cases of prolonged antibiotic use and 18 cases of prolonged hospital stay. Six cases were identified as anastomotic necrosis without any anastomotic dehiscence in the postoperative bronchoscopy examination. These cases presented with productive cough, purulent airway expectoration, fever, and increased white blood cell count. A higher grade of antibiotics and albumin were administered, and bronchoscopy was applied routinely for excretion removal. Bronchoscopy on postoperative day (POD) 8–10 indicated the anastomoses were healed without necrotic or inflammatory appearance. The anastomotic sutures were intact. None of them required additional bronchoscopic treatment or re-operation.
VATS and RATS were mostly performed in the patients with intra-thoracic lesions. Meanwhile, the cervical and cervico-thoracic lesions were usually resected with open surgeries. Most of the patients (49/59, 83.1%) except bronchial lesions (8/9, 88.9%) received non-intubation anesthesia. Thoracic lesion group had longest resection length (3.83 cm in average), while bronchial group and cervical group had shorter mean resection length (2.58 and 2.84 cm, respectively) (Table 2). The patients with cervical or cervico-thoracic lesions received pretracheal release, while all patients with lesions in the thoracic cavity underwent inferior pulmonary ligament releasing. Five cases with thoracic lesions (5/21, 23.8%) and 4 cases (4/9, 44.4%) of carinal resection and reconstruction required hilar and pericardial release. The incidences of prolonged antibiotic use and prolonged hospital stay were slightly higher in thoracic, carinal, and bronchial patients; however, these differences were not significant (Table 2).
Table 2
| Characteristics | Cervical (n=17) | Cervico-thoracic (n=11) | Thoracic (n=21) | Carinal (n=9) | Bronchial (n=9) |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 12 (70.6) | 9 (81.8) | 16 (76.2) | 5 (55.6) | 4 (44.4) |
| Female | 5 (29.4) | 2 (18.2) | 5 (23.8) | 4 (44.4) | 5 (55.6) |
| Release maneuver, n (%) | |||||
| Laryngeal suprahyoid | 2 (11.8) | 0 | 0 | 0 | 0 |
| Pretracheal | 17 (100.0) | 11 (100.0) | 1 (4.8) | 0 | 0 |
| Hilar/pericardial | 0 | 0 | 5 (23.8) | 4 (44.4) | 1 (11.1) |
| IPL | 0 | 0 | 21 (100.0) | 9 (100.0) | 9 (100.0) |
| Anesthesia | |||||
| Intubation | 1 | 0 | 5 | 3 | 7 |
| Non-intubation | 16 | 11 | 16 | 6 | 2 |
| Surgery | |||||
| VATS | 0 | 0 | 14 | 4 | 8 |
| RATS | 0 | 1 | 2 | 1 | 0 |
| Open | 17 | 10 | 5 | 4 | 1 |
| Resection length (cm) | 2.84±1.09 | 3.61±0.71 | 3.83±1.03 | 2.97±0.99 | 2.58±1.33 |
| Pathology, n (%) | |||||
| SQ | 1 (5.9) | 3 (27.3) | 3 (14.3) | 2 (22.2) | 1 (11.1) |
| ACC | 1 (5.9) | 4 (36.4) | 7 (33.3) | 3 (33.3) | 3 (33.3) |
| MEC | 0 | 2 (18.2) | 3 (14.3) | 1 (11.1) | 1 (11.1) |
| Thyroid carcinoma | 4 (23.5) | 0 | 0 | 0 | 0 |
| Other malignant | 0 | 1 (9.1) | 3 (14.3) | 1 (11.1) | 0 |
| Benign | 11 (64.7) | 1 (9.1) | 5 (23.8) | 2 (22.2) | 3 (33.3) |
| Trauma | 0 | 0 | 0 | 0 | 1 (11.1) |
| Complications, n (%) | |||||
| Hoarseness | 2 (11.8) | 1 (9.1) | 2 (9.5) | 1 (11.1) | 0 |
| Prolonged antibiotic | 5 (29.4) | 2 (18.2) | 7 (33.3) | 5 (55.5) | 4 (44.4) |
| Prolonged hospital stay | 4 (23.5) | 1 (9.1) | 5 (23.8) | 3 (33.3) | 3 (33.3) |
| Anastomotic dehiscence | 0 | 0 | 0 | 0 | 0 |
| Anastomotic necrosis | 1 (5.9) | 0 | 2 (9.5) | 1 (11.1) | 2 (22.2) |
| Aspiration | 0 | 0 | 1 (4.8) | 1 (11.1) | 0 |
IPL, inferior pulmonary ligament; VATS, video-assisted thoracic surgery; RATS, robot-assisted thoracic surgery; SQ, squamous cell carcinoma; ACC, adenoid cystic carcinoma; MEC, mucoepidermoid carcinoma.
Most patients (59/67, 88.1%) were discharged without significant short-term complications within 14 days postsurgery. Six patients had hoarseness including two thyroid tumor cases, one cervico-thoracic lesion and three thoracic lesion cases. The symptom is relieved after voice and swallow exercises except two thyroid tumor cases. Eight patients had prolonged postoperative hospital stays ranging from 16 to 38 days. Two patients experienced aspiration and aspiratory pneumonia, presenting with fever, dyspnea, and hypoxemia. They were reintubated and treated with higher grade of antibiotics. The ventilation supports were gradually weaned, and they were finally extubated on POD 16 and 28. After they were transferred to the general ward, they received rehabilitation for swallowing. Three patients experienced anastomotic stenosis, which was confirmed by the postoperative bronchoscopy. Mechanical ventilation, antibiotics, albumin, and bronchoscopy were applied, and the patients were eventually extubated and transferred to the general ward. The predischarge bronchoscopy showed the anastomosis was unobstructed and without purulent excretion but slight edema. The other 3 cases reported as requiring prolonged antibiotic application and prolonged hospital stay included 1 patient with left main bronchus traumatic rupture, 1 with post-intubation tracheoesophageal fistula, and 1 with tracheal squamous cell carcinoma with emphysema. They were eventually discharged without further postoperative complications. The patients were advised to receive cervical and chest CT scan every 3 months and bronchoscopy every 6 months. There was no sign of local tumor recurrence or distant metastasis during the adjuvant therapy and follow-up.
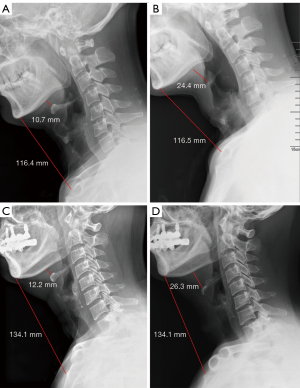
Two thyroid carcinoma patients who received laryngeal and suprahyoid release maneuvers had persistent hoarseness after surgery without any evidence of aspiration. Triiodothyronine (T3), thyroxine (T4), and plasma calcium levels were monitored, and levothyroxine supplemented as required. The 2 patients were on a bilevel positive airway pressure (BiPAP) machine for several days due to mild hypoxia and hypercapnia. No other maneuver-related complication or discomfort was observed or reported. The preoperative and postoperative neck X-ray of both cases show the effect of the suprahyoid release maneuver by 1–1.5 cm (Figure 3).
Discussion
Tension-free anastomosis is the key to successful airway reconstruction. Maneuvers are performed to minimize anastomotic tension, with the simplest being neck flexion. Mulliken and Grillo showed in a cadaveric model that a 4.5 cm length of trachea could be resected without tension with 15 to 35 degrees of neck flexion (14). In addition, the pretracheal plane could be bluntly dissected until the sternal notch, providing some additional, although limited, releasing effects. These simple maneuvers are routinely performed and provide sufficient relief of anastomotic tension for most tracheal operations. However, range of neck motion, age, and cervical pathological condition limit the flexion degree to some extent. Therefore, some advanced release maneuvers are required to facilitate tension-free anastomosis.
In the current study, patients received different release maneuvers, with pretracheal and inferior pulmonary ligament releasing the most commonly performed. Specifically, pretracheal releasing was conducted in all cervical and junctional lesion operations, while inferior pulmonary ligament release was performed in all thoracic lesion cases. These provided extra mobilizations of the trachea or bronchus to achieve tension-free anastomosis. The pulmonary ligament release is usually very well tolerated. Surgeons tend to perform ipsilateral pulmonary ligament release on upper lobectomies. It is generally believed that this maneuver is helpful for better reinflation of the residual lobes, although the evidence is still insufficient. No maneuver-related complications were observed after the pretracheal release maneuver, except for the case of 1 patient with a cervical lesion. The patient underwent tracheal resection and reconstruction with nonintubated anesthesia. After pretracheal release was performed, the patient experienced a drop in oxygen saturation (SpO2) caused by the right pneumothorax, which was subsequently treated by thoracentesis. These outcomes demonstrated the safety and effectiveness of these release maneuvers.
The suprahyoid release is mainly effective in resection of the upper part of the trachea especially in cervical lesions. In the current study, only the cervical lesion patients received suprahyoid release maneuver (15). The Massachusetts General Hospital reported that only 46 of 521 tracheal resection operations for postintubation stenosis involved a laryngeal suprahyoid release. The incidence of laryngeal suprahyoid release increased from 6.4% in primary operations to 29% in re-operations (9). Satisfactory results were reported for these patients. In another series of laryngotracheoplasty resections reported by the same institute, laryngeal release maneuvers were performed in 8.7% of 80 patients (1). The most frequently reported symptoms after suprahyoid release were dysphagia and aspiration. Vocal cord dysfunction was also reported in some studies. In the current cohort, 6 patients including 2 thyroid carcinoma patients who received suprahyoid release maneuvers presented with postoperative hoarseness. After a complete evaluation, the symptom of thyroid tumor patients was considered secondary to thyroid carcinoma invasion of the recurrent nerve and was present pre-operatively in both cases. The hoarseness of other patients was considered as the temporary reaction of aortic arch traction during the trachea mobilization. It is significantly alleviated after voice and swallow exercise. Neither dysphagia nor aspiration was observed in these 2 patients.
The use of hilar and pericardial release maneuvers is encouraged for thoracic tracheal resection operations, and may even be required in patients with concurrent congenital heart disease (16). It works well for moving the bifurcation upwards (17). The possible risks related to these maneuvers are hemorrhage and phrenic nerve injury. The risks are significantly increased in the re-operative cases. Therefore, the dissection should be conducted under direct visualization and close to the inferior pulmonary vein. Mitchell et al. retrospectively investigated 134 carinal resection patients and reported that 37% of them received the hilar release maneuver (18). Anastomotic complications were reported in 23 (17.2%) patients, including stenosis, necrosis, excessive granulation tissue, and bronchial mucosal sloughing. No release maneuver-related complications were reported in this cohort, which indicated the hilar maneuver was relatively reliable and safe. One patient received bilateral hilar and pericardial release maneuvers but had no excessive thoracic drainage or prolonged chest tube placement. Although purulent secretion was noticed under the bronchoscopy, the patient improved after receiving appropriate antibiotics.
Besides the maneuvers described above, longer trachea resection with more complex release maneuvers have been reported. These release maneuvers are especially recommended for re-do trachea resections and longer stenosis and tumor involvements. These operations are generally performed with a median sternotomy. Extensive Hilar release maneuvers are not only performed to release the inferior and superior vein but also include the Pulmonary arteries bilaterally. These include division of broncho-pericardial and trachea-pericardial ligaments (19). Another important release maneuver is pericardiophrenic release which involves dissection of inferior pericardium from the diaphragm. These release maneuvers are reported to be important in extensive resections (20).
Conclusions
Release maneuvers are very helpful in tracheal resection and reconstruction to achieve tension-free anastomosis. Preoperative evaluation is needed to plan the surgical approach and the necessity of release maneuvers. Simple neck flexion and pretracheal dissection provide 4 cm of the trachea for resection. The laryngeal suprahyoid release and hilar pericardial release allow extra length to be resected. These maneuvers are helpful if they are appropriately applied. The study shows that good results can be achieved by application of such maneuvers, with minimal complication and post-operative morbidity.
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-385/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-385/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-385/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2020K-43), the individual consent for the retrospective study was signed and collected from the patients or their legal guardians. The medical data and images would be demonstrated with their official permission.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grillo HC, Mathisen DJ. Primary tracheal tumors: treatment and results. Ann Thorac Surg 1990;49:69-77. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889-96; discussion 1896-7. [Crossref] [PubMed]
- Licht PB, Friis S, Pettersson G. Tracheal cancer in Denmark: a nationwide study. Eur J Cardiothorac Surg 2001;19:339-45. [Crossref] [PubMed]
- Mathisen D. Distal Tracheal Resection and Reconstruction: State of the Art and Lessons Learned. Thorac Surg Clin 2018;28:199-210. [Crossref] [PubMed]
- Auchincloss HG, Mathisen DJ. Tracheal stenosis-resection and reconstruction. Ann Cardiothorac Surg 2018;7:306-8. [Crossref] [PubMed]
- Rob CG, Bateman GH. Reconstruction of the trachea and cervical oesophagus; preliminary report. Br J Surg 1949;37:202-5. [Crossref] [PubMed]
- Belsey R. Resection and reconstruction of the intrathoracic trachea. Br J Surg 1950;38:200-5. [Crossref] [PubMed]
- Salassa JR, Pearson BW, Payne WS. Gross and microscopical blood supply of the trachea. Ann Thorac Surg 1977;24:100-7. [Crossref] [PubMed]
- Broussard B, Mathisen DJ. Tracheal release maneuvers. Ann Cardiothorac Surg 2018;7:293-8. [Crossref] [PubMed]
- Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-92; discussion 492-3. [Crossref] [PubMed]
- Schweiger T, Hoetzenecker K, Klepetko W. Outcome reporting in laryngotracheal surgery: we need functional analysis! Transl Cancer Res 2020;9:2097-8. [Crossref] [PubMed]
- Dedo HH, Fishman NH. Laryngeal release and sleeve resection for tracheal stenosis. Ann Otol Rhinol Laryngol 1969;78:285-96. [Crossref] [PubMed]
- Montgomery WW. Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 1974;99:255-60. [Crossref] [PubMed]
- Mulliken JB, Grillo HC. The limits of tracheal resection with primary anastomosis: further anatomical studies in man. J Thorac Cardiovasc Surg 1968;55:418-21. [Crossref] [PubMed]
- Heitmiller RF. Tracheal release maneuvers. Chest Surg Clin N Am 2003;13:201-10. [Crossref] [PubMed]
- Sengupta A, Murthy RA. Congenital tracheal stenosis & associated cardiac anomalies: operative management & techniques. J Thorac Dis 2020;12:1184-93. [Crossref] [PubMed]
- Rosen FS, Pou AM, Buford WL. Tracheal resection with primary anastomosis in cadavers: the effects of releasing maneuvers and length of tracheal resection on tension. Ann Otol Rhinol Laryngol 2003;112:869-76. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Clinical experience with carinal resection. J Thorac Cardiovasc Surg 1999;117:39-52; discussion 52-3. [Crossref] [PubMed]
- Stamatis G, Freitag L, Greschuchna D. Limited and radical resection for tracheal and bronchopulmonary carcinoid tumour. Report on 227 cases. Eur J Cardiothorac Surg 1990;4:527-32; discussion 533. [Crossref] [PubMed]
- Macchiarini P, Altmayer M, Go T, et al. Technical innovations of carinal resection for nonsmall-cell lung cancer. Ann Thorac Surg 2006;82:1989-97. [Crossref] [PubMed]


