
Metastatic pulmonary carcinoids with EML4-ALK fusion response to ALK inhibitors: two case reports and review of literature
Introduction
Pulmonary carcinoids (PC), including typical (TC) and atypical carcinoids (AC), are low-grade neuroendocrine tumors (NETs) which account for 1–5% of all lung tumors (1). Compared to non-small cell lung cancers (NSCLC), the progress in targeted therapy development in PC is still limited due to the low prevalence of the disease itself and also rare occurrence of Anaplastic lymphoma kinase (ALK) mutations in those tumors. ALK gene rearrangements are present in approximately 5% of NSCLC, but are extremely rare in PC patients (2,3). The screening diagnosis of those mutations is performed by immunohistochemistry. In case of ALK protein positive samples gene translocation must be confirmed by fluorescent in situ hybridization (FISH). In equivocal cases the genetic alteration of ALK can be confirmed by alternative molecular techniques such as next generation sequencing (NGS) or RNA-based PCR methods. NGS and PCR methods enable in-depth understanding of the molecular characteristic of PC, which is extremely useful in case of drug resistance, which is frequent and because of ALK amplification and/or mutation.
Upon administration of ALK inhibitors, acquired resistance is frequent which is mostly due to ALK amplification and/or mutation, and should be tested in recurrent or metastatic tumor samples or circulating nucleic acids. Despite diagnoses of those mutations are extremely rare in PC tumors, such diagnosis may enable further treatment response for targeted therapy.
This report describes the cases of 2 females with metastatic PC presenting an EML4-ALK fusion where targeted therapy was implemented and for a few months those therapies shown a partial response. We present the following article in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-394/rc).
Case presentation
Case 1
A 51-year-old, non-smoking married female, with the history of cancer death in her family (father), was admitted to Tianjin Medical University General Hospital 2 weeks after diagnosis of congestion presented an X-ray 2 weeks before admission. Laboratory tests revealed abnormally elevated levels of neuron-specific enolase (17.78 µg/L; normal value 0.00–16.30 µg/L), progastrin-releasing peptide (281.26 pg/mL; normal value 0.00–63.0 pg/mL), and cytokeratin-19-fragment (4.1 ng/mL; normal value 0.00–3.30 ng/mL). Chest computed tomography (CT) showed a tumor in the left hilum (Figure 1A) and multiple nodules in both lungs. Enhanced magnetic resonance imaging (MRI) revealed abnormal signaling in the brain (Figure 1B). A CT guided lung biopsy was performed. The diagnosis of primary pulmonary AC was confirmed by hematoxylin and eosin staining (Figure 1C) as well as the immunohistochemistry (IHC) positivity of CK7, thyroid transcription factor (TTF-1), synaptophysin, chromogranin A (CgA), carcinoembryonic antigen (CEA), and ki-67 index of 40% (Figure 1D-1F). No p53 expression was found. Given the abnormal radiological findings of multiple lung and brain nodules, the patient was considered to have metastatic pulmonary AC (stage IV), and surgery was not appropriate. To explore the mechanism and potential treatments, we performed NGS (68 cancer gene panel; Burning Rock Biotech, Guangzhou, China) using tissue biopsy. The NGS identified an EML4-ALK rearrangement (E6:A20). The ALK inhibitor alectinib was prescribed at a dose of 600 mg twice a day after patient consent.
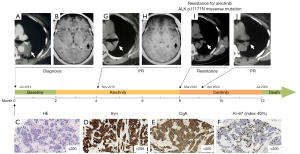
The patient tolerated the treatment well—no grade 2–4 toxicities were noted. After 2 months of treatment with alectinib all of the primary and metastatic lung lesions responded significantly to treatment (Figure 1G), and the metastatic lesions in the brain achieved complete response (Figure 1H). However, the primary lung tumor progressed after further 6 months of alectinib treatment (Figure 1I). An NGS-based blood circulating tumor DNA (ctDNA) test (168 cancer gene panel; Burning Rock, China) was performed. The missense variant of ALK gene p.I1171N in exon 22 was identified besides EML4-ALK variation. Meanwhile, BCL2-like11 (BIM) deletion polymorphism was revealed. Given that ALK gene mutation p.I1171N is the resistant mechanism for alectinib, treatment of alectinib was terminated and ceritinib was initiated at a starting dose of 450 mg/day with food. The subsequent CT scan showed that the primary PC tumor exhibited a remarkable response after 1 month of ceritinib treatment (Figure 1J). However, the patient exhibited poor tolerance to ceritinib treatment, experiencing severe gastrointestinal adverse events of grade 3 nausea and vomiting. The dose of ceritinib was then adjusted to 300 mg/day, which the patient tolerated better, although the symptom of poor appetite persisted. After 3 months treatment of ceritinib, the primary PC tumor exhibited a durable response. Unexpectedly, after 3 months of following further administration of ceritinib, the patient exhibited progressively worsening neurological symptoms (severe disturbance of consciousness amnesia, apathy, and mood disorders). Brain MRI indicated the appearance of new lesions. After a multidisciplinary team discussion, samples of cerebrospinal fluid (CSF) and blood were collected for examination of the antibodies to exclude paraneoplastic syndrome; the results were negative. In addition, CSF and blood were also tested for ctDNA by NGS (168 cancer gene panel; Burning Rock, China). We found a high allelic frequency of EML4-ALK (36.26%) as well as KRAS amplification in CSF, while only a very low allelic frequency (0.05%) of EML4-ALK was detected in blood. The patient died of sudden apnea before the genetic testing results were available. The progression free survival (PFS) of this patient for alectinib and ceritinib was 6 and 4 months, respectively.
Case 2
A 49-year-old non-smoking married female with the history of no previous diseases was admitted to Tianjin Medical University Cancer Institute and Hospital due to severe stomachache lasting 1 week; no treatment had been given from onset to admission. Abdominal CT indicated multiple masses in the liver. A chest CT scan showed a 21-mm nodule in the right upper lobe (Figure 2A). Enhanced MRI showed multiple masses in the brain (Figure 2B). Positron emission tomography-CT (PET-CT) examination exhibited highly F18-fluorodeoxyglucose (FDG) uptake in the lung nodule, liver mass, right adrenal gland, and the bones (Figure 2C). The patient underwent CT guided lung biopsy, and pulmonary AC was diagnosed according to the hematoxylin and eosin (HE) staining, positive immunoreactivity to cytokeratin (CK), CEA, epithelial membrane antigen (EMA), TTF-1, CK7, Synaptophysin, and CgA, as well as the ki-67 index of 30% (Figure 2D-2G). The patient was confirmed as metastatic PC (stage IV). Massive parallel sequencing (654 cancer gene panel; Berry Oncology, Fuzhou, China) indicated that EML4-ALK rearrangement (E13:A20) was present with an allelic frequency of 5.23%, accompanied with TP53 and DOT1L (allelic frequency: 19.68% and 1.01%, respectively). Alectinib at 600 mg twice daily was prescribed after receiving patients’ informed consent. Radiological examinations showed that the patient had significant remission of primary lung tumor (Figure 2H) and brain metastatic lesions (Figure 2I) 9 months after initiation of alectinib. Alectinib treatment was being well tolerated and the treatment is currently ongoing. No severe adverse events have been reported. The PFS is 13 months at present.

Ethical consideration
All procedures performed in this study were in accordance with the ethical standards of Ethics Committee of Tianjin Medical University General Hospital and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Several issues regarding the diagnosis and treatment of this patient for further discussion
Question 1: What is the best treatment method for PC patients with ALK rearrangement, in particular for those with progression of intracranial disease?
Eric H. Bernicker: Still too early to state definitively but if ALK translocation is detected then strong consideration should be given to treatment with alectinib.
Justyna Chalubinska-Fendler: There is no standard treatment, however very scarce but yet promising case presentations showing some data. The debate what kind of ALK inhibitor to use exists, even in patients presenting NSCLC. Crizotinib could be considered but as it did not respond well in patients with NSCLC presenting brain metastases the use of alectinib seems to be the first option. Alectinib is currently widely use in NSCLC patients due to its lower toxicity profile and prevention of brain metastases and progression of existing ones. In case of disease in brain tissue a second-line especially in patients may have a progression in brain the further ALK rearrangement studies should be performed to tailor the treatment in individual way and other treatment such as whole brain radiotherapy or stereotactic surgery for brain lesions should be considered, also for blood-brain barrier “disruption” that may improve drug penetration to brain lesions.
Marc G. Denis: Several inhibitors are now approved in many countries as monotherapy for the treatment of patients with ALK-positive advanced non-small cell lung cancer (NSCLC). Crizotinib was the first-in-class ALK tyrosine kinase inhibitor approved. Due to higher systemic and intracranial efficacy, the second-generation ALK inhibitors alectinib and brigatinib have now replaced crizotinib as standard first-line treatment. A similar approach for PC patients remains to be evaluated.
Question 2: Is ALK inhibitor monotherapy enough for the first-line treatment of advanced PC patient with ALK rearrangement? Which generation ALK inhibitor should be considered as the first choice?
Eric H. Bernicker: Unknown. Trials looking at a combination of sandostatin LAR plus ALK TKI should be looked at.
Marc G. Denis: At present the different ALK inhibitors are indicated as monotherapy in NSCLC. Combination with chemotherapy in PC remains to be evaluated. Based on the different trials performed, there are several possibilities of sequencing ALK inhibitors in NSCLC. But second-generation drugs are the preferred option for most NSCLC patients. Therefore, it is tempting to extrapolate these findings to PC.
Justyna Chalubinska-Fendler: There is no good evidence for it and there is no answer for that question up till now. Apart from standard treatment, where usually due to progression the therapy option is changed one to another after a NET medical board decision, the further trial or therapy with other regimens such as ALK inhibitors—as mentioned in the ANSWER 1 should probably be on the same way as with NSCLC tumors harboring ALK rearrangements so single-agent therapy. There are no robust supportive data both for ALK inhibitor monotherapy as a first line treatment, nor any combination of ALK inhibitors with themselves or with chemotherapy in PC.
Question 3: Is detection of ctDNA necessary for all advanced PC patients to monitor the progression of disease? If not, for which kind of patients should perform detection ctDNA?
Eric H. Bernicker: Again, I don’t think we have enough data to conclusively state that ctDNA should be followed as a routine measure of response. I think baseline ctDNA should be obtained and follow up testing should be done if the radiographic imaging is equivocal or if there is obvious progression and acquired resistance mutations need to be sought.
Marc G. Denis: ctDNA is very useful to detect molecular alterations at diagnosis when tissue sample is not available. At progression on targeted treatment, ctDNA testing may allow to identify resistance mechanisms. Acquired resistance mechanisms to various ALK inhibitors have been described. ALK mutation has been associated with different affinities to ALK inhibitors (4,5). Therefore, it is tempting to identify a specific ALK gene mutation in ctDNA at progression, in order to select the most appropriate subsequent inhibitor to use. This strategy is being evaluated in the NCI-NRG ALK Protocol clinical trial (NCT03737994) On the other hand, since third-generation ALK inhibitors (such as lorlatinib), have been demonstrated, in in vitro models, to be active even in the presence of most resistance mutations, they could also be used without identification of ALK mutation. But ctDNA analysis could also identify “off-target” mechanisms, involving alteration of other genes (HER2, MET, …), that could be targeted by other drugs in the future.
Vincent Thomas de Montpréville: Due to the rarity of advanced PC with ALK rearrangement, I think that each case must presently be considered individually.
Justyna Chalubinska-Fendler: In terms of further ALK rearrangement assessment: ALK inhibitors, acquire resistance frequently which is mostly due to ALK amplification and/or mutation. Diagnostics of these secondary ALK gene alterations must be done from recurrent tumors or circulating nucleic acids.
Discussion
Despite better prognosis of PC than high-grade NETs, including large cell carcinoma (LCNEC) and small cell carcinoma (SCLC), they are still fatal diseases. Little is known of the molecular biological mechanism leading to PC and the treatment strategy for advanced stage PC is not well established. In our case reports, 2 metastatic PC with ALK rearrangement were described and prescribed with ALK inhibitor alectinib. From the literature review, there were 14 lung NETs with ALK arrangement, including 5 AC, 7 LCNEC, and 2 SCLC cases (6-17). We have summarized the clinical features of these cases in the Table 1: there were 11 females and 3 male patients of ages ranging from 32 to 75 years old. Of these 14 patients, 5 cases had a definite smoking history. With the exception of 1 patient with SMC5-ALK and 7 patients without specific variation type due to the detection method used, other patients exhibited EML4-ALK arrangements. The average PFS was 6.9 months for these lung NETs patients receiving ALK inhibitors. PC have a relatively shorter PFS after treatment of ALK inhibitors (7.4 vs. 6.6 months on average) in contrast to LCNEC and SCLC. Shorter PFS may be related to the higher aggressive biological behavior.
Table 1
| Year | Nationality | Sex | Age (y) | Smoking | Primary site | Histology type | Gene mutation | Methods | Stage | Treatment | ALK TKI treatment line | Response | PFS | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | Japan | F | 54 | Yes | Right middle lobe | AC | EML4-ALK | IHC, FISH, multiplex, RT-PCR | IVB | Crizotinib | 1st-line | SD | 5 w | (6) |
| 2020 | China | F | 52 | Unk. | Left lower lung | AC | EML4-ALK | ARMS, IHC | IIB | Surgery, chemotherapy, radiotherapy, crizotinib, ceritinib, alectinib | 4th-line | SD | 16 m | (7) |
| 2016 | Japan | M | 70 | Yes | Left upper lung | AC | ALK rearrangement* | IHC, FISH | IVB | Chemotherapy, crizotinib | 2nd-line | PR | 3 m | (8) |
| 2017 | America | M | 52 | No | Right middle lobe | AC | SMC5-ALK | NGS | IVB | Chemotherapy, radiotherapy, alectinib | 3rd-line | PR | 5 m | (9) |
| 2018 | China | F | 64 | No | Right upper lobe | AC | EML4-ALK | FISH, NGS | IVB | Crizotinib | 1st-line | PR | 12 m | (10) |
| 2014 | Japan | F | 43 | No | Left upper lung | LCNEC | EML4-ALK | IHC, FISH, multiplex, RT-PCR | IVB | Crizotinib | 1st-line | SD | 6 w | (14) |
| 2018 | Japan | F | 75 | No | Left lower lobe | LCNEC | ALK rearrangement* | IHC, FISH | IVB | Chemotherapy, alectinib | 4th-line | PR | 6 m | (12) |
| 2018 | Turkey | F | 69 | No | Unknown | LCNEC | ALK rearrangement* | IHC, FISH | IVA | Chemotherapy, crizotinib | 2nd-line | SD | 9 m | (13) |
| 2020 | Japan | F | 32 | Yes | Left lower lobe | LCNEC | ALK rearrangement* | IHC, FISH | IVB | Alectinib | 1st-line | PR | 11 m | (15) |
| 2021 | France | F | 58 | No | Unk. | LCNEC | ALK rearrangement* | IHC, FISH | IIIA | Chemotherapy, SBRT, alectinib | 3rd-line | PR | 4 m | (11) |
| 2021 | France | F | 74 | No | Unk. | LCNEC | ALK rearrangement* | IHC, FISH | IVA | Chemotherapy, crizotinib, ceritinib, brigatinib | 2nd-line | SD | 11 m | (11) |
| 2021 | France | F | 34 | No | Unk. | LCNEC | ALK rearrangement* | IHC, FISH | IVB | Crizotinib | 1st-line | PD | 4 m | (11) |
| 2013 | Japan | F | 43 | Yes | Left upper lobe | SCLC | EML4-ALK | IHC, RT-PCR | IVA | Chemotherapy | Unk. | PD | 4 m | (17) |
| 2012 | Japan | M | 72 | Yes | Right lower lobe | SCLC + AD | EML4-ALK | IHC | IB | Surgery | Unk. | Unk. | Unk. | (16) |
*, the specific type of ALK rearrangement could not be identified due to the detection method. NETs, neuroendocrine tumors; ALK, anaplastic lymphoma kinase; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; NGS, next generation sequencing; SD, stable disease; PR, partial response; PD, progressive disease; LCNEC, large cell neuroendocrine carcinoma; SCLC, squamous cell lung cancer; AC, atypical carcinoid; AD, adenocarcinoma; RT-PCR, reverse transcription polymerase chain reaction; PFS, progression-free survival; TKI, tyrosine kinase inhibitor; SBRT, stereotactic body radiation therapy; w, weeks; m, months; Unk., unknown.
In the 14 cases (summarized in Table 1), we found that 8 patients were given crizotinib as the first choice of ALK inhibitor, but the results were not very satisfactory. 4 patients showed intracranial metastases after taking crizotinib while the other 2 showed liver metastases and abdominal metastases respectively. A patient underwent disease progression after only 5 weeks of crizotinib treatment. The best response for the fourth patient was only stable disease. As shown in Table 1, the patient who took alectinib with SMC5-ALK variation had significant tumor shrinkage in both the lung and brain, and showed a durable regression until case publication. As we know, the advanced ALK-positive NSCLC patients who took alectinib had a longer (3 times) PFS compared to crizotinib in the ALEX study (18), and particularly showed a greatly reduced rate of brain metastases. As shown in the Table 1, there were 3 AC cases with EML4-ALK fusion and good effectiveness of ALK inhibitors was demonstrated. But the first-line treatment was not alectinib in these 3 AC cases, which was different from us. In future, more cases need to be collected to compare whether the second-generation ALK inhibitor alectinib has a better effect on advanced ALK-positive PC patients compared to crizotinib. Third generation inhibitors must also be evaluated.
Although our cases exhibited a good response to ALK inhibitors, the first female patient only had a 6-month PFS for alectinib, which was much shorter than the median PFS of 38.6 months for alectinib in patients with NSCLC in ALEX study. After the patient exhibited resistance against alectinib, we performed another targeted massive parallel sequencing, and ALK gene exon 22 p.I1171N missense variant as well as BIM deletion polymorphism was revealed. Alectinib treatment was terminated and replaced by ceritinib. The primary lung tumor shrunk again, however brain metastatic disease had significantly progressed according to the brain MRI and NGS results of CSF and blood. It was not immediately obvious why this female had only a 6-month PFS for alectinib treatment and exhibited rapid progression of brain metastases. In a previous study, Christopoulos et al. demonstrated that stage-IV ALK-positive NSCLC patients with TP53 mutations at baseline had a worse overall survival (OS) compared to those with initially TP53 wild-type (44 vs. 62 months in median, P=0.018) (19). Moreover, Christopoulos et al. and Costa et al. also reported that concurrent TP53 variations was associated with a shorter PFS in ALK rearranged NSCLC treated with ALK TKIs (19,20). However, in our study, we did not find a TP53 alteration in case 1. We speculate that the intrinsic histologic feature of PC determined the poor prognosis to ALK inhibitors. In addition, it is possible that BIM deletion polymorphism was involved in the poor PFS. A previous report showed that BCL2L11 was vital to regulate cell apoptosis (21). It was also reported that due to the germline deletion polymorphism of BIM, the expression of the pro-apoptotic BCL2 homology domain 3 (BH3) is impaired, which can be considered a possible factor leading to epidermal growth factor receptor (EGFR) TKI resistance in NSCLC patients with EGFR mutations (22). Moreover, a study has shown that the germline BIM deletion may be related to poor efficacy of TKIs such as crizotinib and imatinib (23). We proposed that the BIM deletion polymorphism may have led to the shorter PFS of the first female patient treated with alectinib, however this warrants further investigations in larger cohorts.
As the first report documenting the missense variation of ALK gene p.I1171N in exon 22 in a PC patient with EML4-ALK after alectinib resistance, we used ceritinib which was sensitive to exon 22 p.I1171N missense variation to achieve a patient benefit of 4-month PFS, indicating ceritinib was still effective after drug resistance due to p.I1171N in PC patients. Our report has provided not only feasible treatment for advanced PC patients with ALK rearrangement, but also a strategy after ALK inhibitor resistance due to p.I1171N in PC patients with ALK rearrangement. This led to administration of ceritinib. Unfortunately, the first patient performed a 4-month PFS only after taking ceritinib and then intracranial disease progressed and resulted in death. That situation indicates that ceritinib response to the primary tumor may differ from response of metastatic, especially intracranial disease. The additional detection through ctDNA in blood or CSF may help to differ the progression from pseudoprogression or paraneoplastic syndrome.
In conclusion, lung NETs with ALK rearrangement are extremely rare and there is no standard treatment for advanced patients so far. Based on our literature review and case reports we have presented that there may be a room for diagnosing ALK rearrangements in PC. Even if those are rare in this rare group of lung tumors, ALK inhibitors could be an effective treatment strategy for some patients, leading to at least a partial response. Moreover, we present the first in the literature missense variant of ALK gene p.I1171N in exon 22 which was diagnosed in one of the patients with PC presented in this manuscript.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This study was funded by the National Natural Science Foundation of China (Nos. 82172620 and 82172776), Tianjin Science and Technology Plan Project (No. 19ZXDBSY00060), and Tianjin Key Medical Discipline (Specialty) Construction Project.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-394/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-394/coif). MGD received grants from Takeda and Blueprint Medicines, honoraria for lectures from Pfizer and BMS, support for attending meetings from Pfizer, Takeda and AstraZeneca, and honoraria for advisory boards from Amgen, AstraZeneca, Takeda, Janssen and Daiichi-Sankyo. EHB received fess for serving on advisory boards for Astra Zeneca, Blueprint medicine and Guardant health. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of Ethics Committee of Tianjin Medical University General Hospital and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Steuer CE, Behera M, Kim S, et al. Atypical carcinoid tumor of the lung: a surveillance, epidemiology, and end results database analysis. J Thorac Oncol 2015;10:479-85. [Crossref] [PubMed]
- Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res 2015;21:2227-35. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Horn L, Whisenant JG, Wakelee H, et al. Monitoring Therapeutic Response and Resistance: Analysis of Circulating Tumor DNA in Patients With ALK+ Lung Cancer. J Thorac Oncol 2019;14:1901-11. [Crossref] [PubMed]
- Fukuizumi A, Akagi K, Sakai H. A Case of Atypical Carcinoid: Harboring Variant 3a/b EML4-ALK Rearrangement. J Thorac Oncol 2015;10:e104-6. [Crossref] [PubMed]
- Liu N, Wang J, Fu X, et al. A case of primary pulmonary atypical carcinoid with EML4-ALK rearrangement. Cancer Biol Ther 2020;21:12-6. [Crossref] [PubMed]
- Nakajima M, Uchiyama N, Shigemasa R, et al. Atypical Carcinoid Tumor with Anaplastic Lymphoma Kinase (ALK) Rearrangement Successfully Treated by an ALK Inhibitor. Intern Med 2016;55:3151-3. [Crossref] [PubMed]
- Wang VE, Young L, Ali S, et al. A Case of Metastatic Atypical Neuroendocrine Tumor with ALK Translocation and Diffuse Brain Metastases. Oncologist 2017;22:768-73. [Crossref] [PubMed]
- Zheng Q, Zheng M, Jin Y, et al. ALK-rearrangement neuroendocrine carcinoma of the lung: a comprehensive study of a rare case series and review of literature. Onco Targets Ther 2018;11:4991-8. [Crossref] [PubMed]
- Doubre H, Fraboulet S, Longchampt E, et al. ALK Rearrangement in Lung Neuroendocrine Neoplasms: Case Series of Non-Asian Patients With Response to ALK Inhibitors. Clin Lung Cancer 2021;22:e686-90. [Crossref] [PubMed]
- Hayashi N, Fujita A, Saikai T, et al. Large Cell Neuroendocrine Carcinoma Harboring an Anaplastic Lymphoma Kinase (ALK) Rearrangement with Response to Alectinib. Intern Med 2018;57:713-6. [Crossref] [PubMed]
- Hoton D, Humblet Y, Libbrecht L. Phenotypic variation of an ALK-positive large-cell neuroendocrine lung carcinoma with carcinoid morphology during treatment with ALK inhibitors. Histopathology 2018;72:707-10. [Crossref] [PubMed]
- Omachi N, Shimizu S, Kawaguchi T, et al. A case of large-cell neuroendocrine carcinoma harboring an EML4-ALK rearrangement with resistance to the ALK inhibitor crizotinib. J Thorac Oncol 2014;9:e40-2. [Crossref] [PubMed]
- Tashiro T, Imamura K, Tomita Y, et al. Heterogeneous Tumor-Immune Microenvironments between Primary and Metastatic Tumors in a Patient with ALK Rearrangement-Positive Large Cell Neuroendocrine Carcinoma. Int J Mol Sci 2020;21:9705. [Crossref] [PubMed]
- Toyokawa G, Taguchi K, Ohba T, et al. First case of combined small-cell lung cancer with adenocarcinoma harboring EML4-ALK fusion and an exon 19 EGFR mutation in each histological component. J Thorac Oncol 2012;7:e39-41. [Crossref] [PubMed]
- Toyokawa G, Takenoyama M, Taguchi K, et al. An extremely rare case of small-cell lung cancer harboring variant 2 of the EML4-ALK fusion gene. Lung Cancer 2013;81:487-90. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Christopoulos P, Dietz S, Kirchner M, et al. Detection of TP53 Mutations in Tissue or Liquid Rebiopsies at Progression Identifies ALK+ Lung Cancer Patients with Poor Survival. Cancers (Basel) 2019;11:124. [Crossref] [PubMed]
- Costa DB. TP53 mutations are predictive and prognostic when co-occurring with ALK rearrangements in lung cancer. Ann Oncol 2018;29:2028-30. [Crossref] [PubMed]
- Concannon CG, Tuffy LP, Weisová P, et al. AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J Cell Biol 2010;189:83-94. [Crossref] [PubMed]
- Yuan J, Li B, Zhang N, et al. Clinical Implications of the BIM Deletion Polymorphism in Advanced Lung Adenocarcinoma Treated With Gefitinib. Clin Lung Cancer 2018;19:e431-8. [Crossref] [PubMed]
- Ng KP, Hillmer AM, Chuah CT, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med 2012;18:521-8. [Crossref] [PubMed]


