
Intraoperative methods for wrapping anastomoses after airway reconstruction: a case series
Introduction
Airway resection and reconstruction is a feasible method to treat airway diseases (1,2). The procedure is challenging, requiring delicate suture skills, accurate reconstruction mode selection, and airway surveillance, and complications such as anastomotic stenosis or fistula can lead to detrimental outcomes and poor prognosis (3,4). Anastomosis management is of vital importance, and the anastomotic buttress has always been an important concern. To protect the anastomosis, surgeons wrap it with various options including mediastinal pleural flap, anterior cervical muscle, sternocleidomastoid, thymus flap, intercostal muscle flap, biological patch, prepericardial fat, thyroid gland, pectoralis major, and omental flap (5-9). These coverages provide additional layers of protection against anastomotic failure and preserve the continuity of the airway in case of small dehiscence (10). Thus, it is essential to treat each patient with sufficient flexibility and take into account the difficulty of obtaining, the anatomic position, and the blood supply conditions of the wrapping materials. It is important to choose the safest and most easy-processing wrapping materials under different situations. However, research exploring the principles of how to choose materials is rare. This article explores tracheobronchial resection and reconstruction followed by anastomosis wrapping in a series of cases and summarizes the experience of using different wrapping materials. We aim to demonstrate and evaluate the buttress materials of anastomosis in many aspects, including preoperative characteristics, intraoperative features, and the incidence of postoperative complications. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-406/rc).
Methods
Patient characteristics
This retrospective series report recruited 62 consecutive patients who underwent airway resection and reconstruction for diseases at the First Affiliated Hospital of Guangzhou Medical University between January 2019 and September 2021. Bronchoscopy was performed preoperatively on all patients to evaluate the lesion and histopathological type. The lesions were distributed at the cervical trachea, thoracic trachea, carina, main bronchi, and secondary carina, and the preoperative diagnoses included stenosis after tracheal postintubation, primary tumor, metastatic tumor, and thoracic trauma (Table 1). Postoperative computed tomography (CT) scans, bronchoscopy, complication rates, and survival rates were used to evaluate the outcome of surgery. The clinical data were manually accrued retrospectively from individual electronic medical records and hospital charts.We followed up patients by telephone and outpatient, and the last follow-up date was March 2022. We evaluate the efficacy and safety outcomes of wrapping materials based on the postoperative complications of the participants, the surgical practice of surgeon, and the intrinsic properties of the materials.
Table 1
| Variables | Results |
|---|---|
| Age (years), median [IQR] | 42.0 [32–57] |
| Gender, n (%) | |
| Male | 45 (72.6) |
| Female | 17 (27.4) |
| BMI (kg/m2), median (IQR) | 23.47 (21.65–25.37) |
| Comorbidities, n (%) | |
| Cardiac disease | 1 (1.6) |
| Diabetes | 2 (3.2) |
| Hypertension | 3 (4.8) |
| Emphysema | 5 (8.1) |
| Hypertension & diabetes | 2 (3.2) |
| Hypertension & cardiac disease | 1 (1.6) |
| History of other neoplasms | 4 (6.5) |
| Previous airway procedures, n (%) | 11 (17.7) |
| Neoadjuvant therapy, n (%) | |
| Neoadjuvant chemotherapy | 3 (4.8) |
| Neoadjuvant immunotherapy and chemotherapy | 4 (6.5) |
| Neoadjuvant chemoradiotherapy | 2 (3.2) |
| Preoperative diagnoses, n (%) | |
| Stenosis after tracheal procedures | 8 (12.9) |
| Primary tumor | 49 (79.0) |
| Metastatic tumor | 3 (4.9) |
| Thoracic trauma | 2 (3.2) |
BMI, body mass index.
The study was conducted following the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2020K-43), and individual consent for this retrospective analysis was waived.
Utilization of buttress
Variables
Anastomotic wrapping was defined as the overlaying of different tissue structures or biomaterials on the anastomosis of tracheal reconstruction (Figure 1).
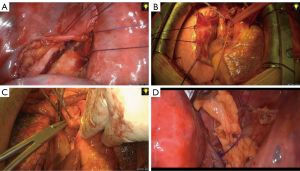
Mediastinal pleural flap
A mediastinal pleura flap was obtained with thoracoscopic assistance. An anastomotic plane between the pleura and the trachea was dissected. The pleura adjacent to the trachea was preserved and trimmed to a flap. The flap was then folded around the tracheal anastomosis. The interrupted sutures (4-0 Vicryl Ethicon Inc., Cornelia, GA, USA) were placed to approximate the flap and the anastomosis.
Anterior cervical muscles or thyroid gland
As both the anterior cervical muscles and the thyroid gland have a good blood supply and are just above the upper trachea anastomosis, they can be directly sewn on the anastomosis with sutures to prevent severe complications such as a trachea carotid artery fistula. Both lobes of the thyroid gland would be dissected from the strap muscles and the trachea to facilitate the anastomosis. The blood supply of the thyroid gland and parathyroid gland should be preserved. The strap muscles would be dissected and trimmed to a flap. The interrupted sutures (4-0 Vicryl Ethicon Inc., Cornelia, GA, USA) were placed to approximate the flap and the anastomosis. The sternocleidomastoid muscle could be used as the alternative if the strap muscles are unavailable, especially in the secondary operation cases.
Thymus flap
After reserving the vessel supply of the thymic arteries, the thymus was then placed and wrapped around the trachea anastomosis via a transverse mattress suture using 4-0 Vicryl stitches.
Intercostal muscle flap
The intercostal muscle on the level of the trachea anastomosis was selected and dissociated to 5 cm beside the spine from the distal end to the proximal end. During the process, attention was paid to avoid injuring the paravertebral vein and intercostal artery. Finally, the intercostal muscle was embedded at the anastomosis, which was approximated onto the tracheal wall with 4-0 Vicryl interrupted sutures.
Prepericardial fat
The pedicled fat pad was formed by dissociating the prepericardial fat pad from the pericardium. The anastomosis was wrapped around, and the prepericardial fat was fixed at the anastomosis with 4-0 VIRCYL sutures to prevent its postoperative displacement.
Pectoralis major
The right pectoralis major, pedicled with the thoracoacromial artery, enters the mediastinum through the second intercostal space and was wrapped around the trachea anastomosis with 4-0 Vicryl sutures. Then the pectoralis major was then fixed on the surrounding fascia to prevent the flap from retracting.
Omental flap
The right gastroepiploic artery was protected and the greater omental flap off the lower margin of the stomach and transverse colon was released. The omental flap was then placed under the right diaphragm for subsequent retrieval. After carinal resection and reconstruction, the omental flap was retrieved through a 5 cm opening in the diaphragm without any torsion and wrapped around the anastomosis with 4-0 Vicryl sutures. The diaphragm was then carefully closed with interrupted 1-0 silk, without strangulating the flap.
Statistical analysis
Data were analyzed as proportions, means, or medians according to their nature using SPSS software (version 25).
Results
The clinical characteristics of all 62 patients were recorded. The median age of these patients was 42 years (IQR, 32–57 years), there were 45 males and 17 females, and comorbidities were found in 18 (29.0%). Tracheal procedures, such as endotracheal intubation, tracheostomy, tracheal expansion, and stent implantation, were performed in 11 (17.7%) before surgery, and nine patients received neoadjuvant therapy (Table 1). Cervical tracheal resection and reconstruction were performed in 18/62 (29.0%) patients, thoracic tracheal resection and reconstruction in 27/62 (43.6%), carinal resection and reconstruction in 8/62 (12.9%), and secondary carinal and main bronchial resection and reconstruction in 9/62 (14.5%). The resected distance from the lesion was from 0.5 to 1.0 cm. The reconstruction procedures were decided by the actual surgical condition, using continuous suture with 2-0 Prolene or 3-0 Prolene (Ethicon Inc., Cornelia, GA, USA), followed by 4-0 Vicryl interrupted sutures.
Anastomotic buttress materials included the following procedures: 24 mediastinal pleural flap (24/62, 38.7%), 14 anterior cervical muscle (14/62, 22.6%), 2 sternocleidomastoid (2/62, 3.2%), 12 thymus flap (12/62, 19.4%), 2 intercostal muscle flap (2/62, 3.2%), 2 biological patch (2/62, 3.2%), 1 prepericardial fat (1/62, 1.6%), 1 thyroid gland (1/62, 1.6%), 2 pectoralis major (2/62, 3.2%), and 2 omental flap (2/62, 3.2%). All anastomotic buttress materials were wrapped by a continuous transverse mattress suture technique using 4-0 Vicryl before the chest was closed with chest drainages after confirming no bleeding and air leak. The final postoperative histological examination showed squamous cell carcinoma in 11 patients (17.7%), adenoid cystic carcinoma in 16 (25.8%), chronic inflammation in 10 patients (16.1%), mucoepidermoid carcinoma in 8 (12.9%), tracheal glomus tumor in 4 patients (6.5%), thyroid cancer in 3 patients (4.8%), and other histological types in 10 patients (16.1%). Surgical approaches and postoperative complications were elaborate, and no 90-day mortality was observed (Table 2). The median postoperative hospitalization time was 7 days (range, 4–28 days). Bronchoscopy was performed a week after surgery and showed no severe anastomosis-related complications, such as dehiscence or separation. A follow-up for 6 months was conducted and all patients were alive postoperatively. Tracheomalacia stenosis postoperatively occurred in 3 patients and they were subsequently treated with an endotracheal stent. One patient had tumor recurrence 3 months after surgery and received adjuvant chemotherapy.
Table 2
| Materials | Surgical methods | Approaches | Short-term anastomotic complication | Long-term anastomotic complications | Postoperative status after 6 months |
|---|---|---|---|---|---|
| Mediastinal pleural flap | VATS thoracic trachea [14] | Right thoracic approach | No | Tumor recurrence [1] | Alive |
| RATS thoracic trachea [2] | Da Vinci XI right thoracic approach | No | No | ||
| VATS carinal [2] | Right thoracic approach | No | No | ||
| VATS right main bronchus [2] | Thoracic approach | No | No | ||
| VATS left main bronchus [4] | Thoracic approach | No | No | ||
| Anterior cervical muscle (including sternohyoid) | Cervical trachea [13] | Neck dissection | No | Postoperative tracheomalacia stenosis [1] | Alive |
| Thoracic trachea [1] | Sternotomy | No | Postoperative tracheomalacia stenosis [1] | ||
| Sternocleidomastoid | Cervical trachea [1] | Neck dissection | No | No | Alive |
| VATS cervical trachea [1] | Left thoracic approach & neck dissection | No | No | ||
| Thymus flap | Cervical trachea [2] | Neck dissection Neck dissection & sternotomy | No | Postoperative tracheomalacia stenosis [1] | Alive |
| Thoracic trachea [7] | Sternotomy | No | No | ||
| Carinal [3] | Sternotomy | No | No | ||
| Intercostals muscle flap | RATS carinal [1] | Da Vinci XI right thoracic approach | No | No | Alive |
| VATS C3 carinal and right main bronchus [1] | Right thoracic approach | No | No | ||
| Biological patch | VATS carinal [1] | Right thoracic approach | No | No | Alive |
| VATS thoracic trachea [1] | Right thoracic approach | No | No | ||
| Prepericardial fat | RATS left main bronchus [1] | Da Vinci XI left thoracic approach | No | No | Alive |
| Thyroid gland | Cervical trachea [1] | Neck dissection | No | No | Alive |
| Pectoralis major | Thoracic trachea [2] | Sternotomy | No | No | Alive |
| Omental flap | VATS carinal [1] | Right thoracic approach | No | No | Alive |
| VATS right main bronchus [1] | Right thoracic approach | No | No |
Numbers in brackets represent the operation volume. VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracic surgery.
Discussion
Airway resection and reconstruction remain challenging procedures owing to the technical complexity and postoperative complications related to the airway anastomosis. Anastomotic dehiscence, stenosis, and fistula are relatively common anastomotic complications and require expert postoperative management and intraoperative anastomosis wrapping is a potential method to reduce anastomosis complications (5). In the 1970s, Dr. Grillo and colleagues developed techniques to protect the tracheal anastomosis using autograft methods such as a pleura flap to wrap the anastomosis (11). Anastomotic wrapping provides an additional layer of barriers to protect the anastomosis, separates it from the artery and other structures to avoid fistula formation, and aids in revascularization thus, improving anastomotic healing.
A variety of wrapping materials for tracheobronchial resection and reconstruction have been reported, and the options introduced in our article are also very diverse, including the anterior cervical muscle group, sternocleidomastoid muscle, thyroid, mediastinal pleura, thymus flap, pedicled prepericardial fat pad intercostal muscle flap, omental flap, pectoralis major muscle flap, and biological patch. The anatomical position, area, invasiveness, accessibility, blood supply, and plasticity are different among each of these, based on the experience of surgeons (Table 3).
Table 3
| Materials | Distance | Plasticity | Blood supply | Invasiveness | Accessibility | Technical practicability |
|---|---|---|---|---|---|---|
| Mediastinal pleural flap | + | + | + | ++ | +++ | +++ |
| Anterior cervical muscle (including sternohyoid) | ++ | ++ | ++ | + | +++ | +++ |
| Sternocleidomastoid | ++ | ++ | ++ | + | +++ | +++ |
| Thymus flap | ++ | +++ | + | + | +++ | ++ |
| Intercostal muscle flap | +++ | ++ | ++ | +++ | ++ | + |
| Biological patch | − | ++ | − | − | +++ | ++ |
| Prepericardial fat pad | + | + | + | + | ++ | ++ |
| Thyroid gland | + | + | ++ | + | +++ | +++ |
| Pectoralis major | +++ | +++ | +++ | +++ | + | + |
| Omental flap | +++ | +++ | +++ | +++ | + | + |
+++, highly present; ++, moderately present; +, mildly present; −, absent.
Anastomosis after cervical trachea resection and reconstruction is usually wrapped by the anterior cervical muscle group because this group is anterior to the cervical trachea and located adjacent to the anastomosis. Of these, the sternohyoid muscle is most commonly used, although the sternocleidomastoid muscle is an alternative material for patients with an abnormal structure of the anterior cervical muscle group. Mobilizing the sternocleidomastoid muscle may lead to a limitation of rotative activity, while the thyroid will be used when the anastomosis is directly under it or close to it.
Incision of the mediastinal pleura is a step for airway resection and reconstruction, especially when a right-sided VATS approach for thoracic tracheal resection is used. At the end of the reconstruction, using the mediastinal pleura to wrap the anastomosis is conveniently located and mobile, and can provide extra protection for the anastomosis and set a barrier between the trachea and other structures to prevent from the complications such as tracheo-innominate artery fistula and tracheoesophageal fistula. Some articles suggest the blood supply of the mediastinal pleura is limited, and it cannot effectively prevent the occurrence of complications (10). From our experience, wrapping the trachea or bronchus with mediastinal pleura won’t lead to more complications. To be on the safe side, it would be better for patients with diabetes mellitus, neoadjuvant therapy or hypoproteinemia to use materials with rich blood supply.
The thymus flap is located directly posterior to the sternum and is useful for covering the tracheobronchial anastomosis from the cervicothoracic boundary to the carina when a sternotomy is performed. The median sternotomy divides loose adhesions of peri-thymic tissue and improves mobilization of the thymus. It has a rich vascular supply, high tensile strength, and sufficient volume compared to a thin pleural flap, so it is mainly used for long-segment tracheal or carinal reconstruction. The thymus, which is adjacent to the trachea, can be embedded easily and safely in the thoracic tracheal anastomosis and placed between it and blood vessels after full dissociation. However, there are some disadvantages to using the thymus flap. As the thymus sometimes presents an atrophied fibrofatty tissue and has less volume for patch reinforcement in adulthood (12), preoperative CT evaluation of thymus volume is recommended. Interestingly, most patients have a sufficient volume of thymus flap to wrap the anastomosis clinically, despite a possible negative CT appearance of sufficient thymus gland, and no anastomotic complications were observed in our cohort.
The omental flap has the properties of anti-inflammation, regeneration, and good plasticity. It possesses a rich blood supply and inflammatory factors, which are beneficial to resist infection, and potentially promoteanastomotic healing (13). Therefore, it is often used in complex cases of trachea reconstruction, such as in patients after neoadjuvant therapy (14). While these characteristics render the omental flap an ideal material for tracheobronchial anastomosis wrapping, omentopexy has the disadvantage of requiring an additional invasive abdominal exploration, which requires the use of complex laparoscopy and the assistance of a general surgeon. Due to its rich blood supply, high-risk patients with diabetes or those who have received neoadjuvant treatment can benefit from the use of an omental flap to minimize the risk of anastomotic complications. In this study, two patients who received neoadjuvant treatment (1 chemoradiotherapy, 1 chemotherapy and immunotherapy) were wrapped with an omental flap, and neither had postoperative complications.
Affluently vascularized intercostal muscle flaps promote tracheobronchial revascularization after tracheobronchial resection and reconstruction early in the postoperative period. Obtaining a pedicled intercostal muscle flap is relatively difficult under VATS, and ensuring the adequate length of the flap to wrap around the anastomosis is necessary. However, the procedure of making an intercostal muscle flap is invasive and potentially triggers serious postoperative pain due to the damage to the intercostal nerve (15), and for these reasons, the procedure is not regularly used in wrapping the tracheobronchial anastomosis. While no early complications were observed in this study using intercostal muscle flaps under VATS, previous reports suggest it may cause ossification, although the clinical implications of this are unclear (16).
The creation of a pedicled prepericardial fat wrap is easy under VATS (17). Pedicled prepericardial fat is generally used for wrapping the lower trachea, the carina, and the main bronchus, compared with the omental flap, and the use of the former obviates the necessity to perform abdominal surgery. The use of pedicled prepericardial fat to reinforce the anastomosis is proven and is a good choice for patients who have high-risk factors. Pedicled prepericardial fat can release more angiogenin and other growth factors related to anastomotic healing (18), and its use has been satisfactory in enhancing tracheal healing as well as adding more bulk between the separation of the aorta and trachea. Pedicled prepericardial fat is flexible, light, and exerts no extra tension on the tracheobronchial anastomosis with the churning motion of the heart. While it is such a well-vascularized tissue and easily obtained, only one patient had pedicle prepericardial fat to wrap around the tracheobronchial anastomosis in our study. This is because the volume of prepericardial fat required in these procedures only exists in patients with relatively high BMI, which the thymus flap can meet the demand of wrapping in most situations.
When a long-segment trachea resection and reconstruction is performed and the anastomosis is under extremely high tension, other wrapping materials like a pectoralis major muscle flap or a biological patch will be used. The pectoralis major muscle flap is thick, robust, and large, and wrapping with it greatly reduces the risk of anastomotic dehiscence. However, obtaining a pectoralis major muscle flap is quite invasive and the operation time is longer often requiring a plastic reconstructive surgeon, so it is an uncommon wrapping method. Whilst in cases where an insufficient quantity of pectoralis major muscle exists, a biological patch may be a substitute. A biological patch covers a variety of options and in particular, autologous stem cell patches such as those using human induced pluripotent stem cells (IPS cells). However, how to achieve revascularization with a biological patch is an unsolved problem.
Multiple factors influence anastomotic healing, such as diabetes, technical skill, hypoproteinemia, neoadjuvant therapy, and infection (19-21). Previous studies have pointed out that diabetes mellitus is an independent risk factor for poor anastomotic healing (22), although Sfyridis et al. suggested the use of intercostal muscle flaps to wrap the bronchial stump of diabetic patients after lobectomy or pneumonectomy could reduce the occurrence of bronchopleural fistula (23). In the present study, all diabetic patients had no postoperative complications. Neoadjuvant chemoradiotherapy potentially decreases the blood supply of the trachea and bronchi, resulting in poor anastomotic healing. Chen et al. demonstrated that wrapping the carinal anastomosis with an omental flap was useful for patients receiving neoadjuvant therapy (14), and in the present study, patients who received neoadjuvant therapy had no postoperative complications, including two patient who received chemoradiotherapy.
In conclusion, various anastomosis wrapping methods and procedures are used in tracheobronchial resection and reconstruction. Intraoperative methods are selected according to their characteristics, anatomical position, and technical difficulty. This study indicated that appropriate surgical buttresses for wrapping anastomoses are legitimate alternatives in minimizing the risk of anastomotic complications according to our center’s large volume of complex cases. However, as the results of this study are limited by a retrospective study conducted at a single institution, a randomized trial of multiple centers is required.
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-406/rc
Data Sharing Statement: Available at: https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-406/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-406/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted following the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2020K-43), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grillo HC. Development of tracheal surgery: a historical review. Part 1: Techniques of tracheal surgery. Ann Thorac Surg 2003;75:610-9. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Clinical experience with carinal resection. J Thorac Cardiovasc Surg 1999;117:39-52; discussion 52-3. [Crossref] [PubMed]
- Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731-9. [Crossref] [PubMed]
- Wright CD, Li S, Geller AD, et al. Postintubation Tracheal Stenosis: Management and Results 1993 to 2017. Ann Thorac Surg 2019;108:1471-7. [Crossref] [PubMed]
- Anderson TM, Miller JI Jr. Use of pleura, azygos vein, pericardium, and muscle flaps in tracheobronchial surgery. Ann Thorac Surg 1995;60:729-33. [Crossref] [PubMed]
- Deng D, Xu F, Liu J, et al. Clinical application of pedicled thoracoacromial artery perforator flaps for tracheal reconstruction. BMC Surg 2020;20:299. [Crossref] [PubMed]
- Sihag S, Wright CD. Prevention and Management of Complications Following Tracheal Resection. Thorac Surg Clin 2015;25:499-508. [Crossref] [PubMed]
- Levashev YN, Akopov AL, Mosin IV. The possibilities of greater omentum usage in thoracic surgery. Eur J Cardiothorac Surg 1999;15:465-8. [Crossref] [PubMed]
- Udelsman BV, Eaton J, Muniappan A, et al. Repair of large airway defects with bioprosthetic materials. J Thorac Cardiovasc Surg 2016;152:1388-97. [Crossref] [PubMed]
- Taghavi S, Marta GM, Lang G, et al. Bronchial stump coverage with a pedicled pericardial flap: an effective method for prevention of postpneumonectomy bronchopleural fistula. Ann Thorac Surg 2005;79:284-8. [Crossref] [PubMed]
- Grillo HC. Reconstruction of the trachea. Experience in 100 consecutive cases. Thorax 1973;28:667-79. [Crossref] [PubMed]
- Fukumoto Y, Matsunaga T, Shishido Y, et al. Successful repair using thymus pedicle flap for tracheoesophageal fistula: a case report. Surg Case Rep 2018;4:49. [Crossref] [PubMed]
- Shrager JB, Wain JC, Wright CD, et al. Omentum is highly effective in the management of complex cardiothoracic surgical problems. J Thorac Cardiovasc Surg 2003;125:526-32. [Crossref] [PubMed]
- Chen J, Ang KL, Wang C, et al. Minimally Invasive Carinal Reconstruction Using Bronchial Flap and Omental Flap Reinforcement. Ann Thorac Surg 2022;113:e255-7. [Crossref] [PubMed]
- Deeb ME, Sterman DH, Shrager JB, et al. Bronchial anastomotic stricutre caused by ossification of an intercostal muscle flap. Ann Thorac Surg 2001;71:1700-2. [Crossref] [PubMed]
- Maniwa T, Saito Y, Saito T, et al. Ossification does not cause any complication when a bronchial stump is reinforced with an intercostal muscle flap. Eur J Cardiothorac Surg 2009;35:435-8. [Crossref] [PubMed]
- Watanabe A, Abe T, Yamauchi A, et al. Reinforcement of a bronchial stump in VATS lobectomy. Thorac Cardiovasc Surg 2000;48:242-3. [PubMed]
- Shoji F, Yano T, Miura N, et al. Pericardial fat pad tissue produces angiogenic factors for healing the bronchial stump. Interact Cardiovasc Thorac Surg 2011;13:271-5. [Crossref] [PubMed]
- Li SJ, Fan J, Zhou J, et al. Diabetes Mellitus and Risk of Bronchopleural Fistula After Pulmonary Resections: A Meta-Analysis. Ann Thorac Surg 2016;102:328-39. [Crossref] [PubMed]
- Yamamoto R, Tada H, Kishi A, et al. Effects of preoperative chemotherapy and radiation therapy on human bronchial blood flow. J Thorac Cardiovasc Surg 2000;119:939-45. [Crossref] [PubMed]
- Storelli E, Tutic M, Kestenholz P, et al. Sleeve resections with unprotected bronchial anastomoses are safe even after neoadjuvant therapy. Eur J Cardiothorac Surg 2012;42:77-81. [Crossref] [PubMed]
- Li SJ, Fan J, Zhou J, et al. Diabetes Mellitus and Risk of Bronchopleural Fistula After Pulmonary Resections: A Meta-Analysis. Ann Thorac Surg 2016;102:328-39. [Crossref] [PubMed]
- Sfyridis PG, Kapetanakis EI, Baltayiannis NE, et al. Bronchial stump buttressing with an intercostal muscle flap in diabetic patients. Ann Thorac Surg 2007;84:967-71. [Crossref] [PubMed]


