
Immune checkpoint inhibitor (ICI)-based treatment beyond progression with prior immunotherapy in patients with stage IV non-small cell lung cancer: a retrospective study
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1) and programmed death-ligand 1 (PD-L1) have greatly improved the survival of advanced non-small cell lung cancer (NSCLC) (1-3). Despite a significant survival advantage for patients with advanced NSCLC treated with immunotherapy, disease progression is still common and often inevitable (4). The subsequent treatment options of patients who experience ICI treatment failure consist mostly of traditional chemotherapy, with median progression-free survival (PFS) ranging from 2.8 to 4.5 months and median overall survival (OS) ranging from 6.8 to 7.5 months (5-7). Clinically, there is still much room for significant improvement in the subsequent treatment of advanced NSCLC patients who have failed with immunotherapy.
The application of ICI treatment beyond progression (TBP) with prior immunotherapy among patients with advanced NSCLC has attracted attention. According to post-hoc analyses of Checkmate 153 and Keynote 010, patients who received a second PD-1 inhibitor course after prior ICI treatment failure can still respond again (8,9). But highly selected patients in clinical research can not necessarily fully reflect the real-world clinical setting. According to some retrospective studies, patients who received ICI TBP were reported to be associated with better clinical outcomes compared to the non-TBP group under real-world conditions (10-12). However, those prospective studies with limited samples reported different outcomes about ICI TBP after prior immunotherapy, some with a median PFS of less than 3 months (13,14), whereas others with more than 5 months (15,16). Outside of clinical trial settings, other retrospective studies demonstrated that median PFS varied from 1.7 to 4.4 months, but more comprehensive statistical analyses were limited due to smaller sample sizes (17-24). Overall, the role of ICI TBP with prior immunotherapy is still not fully clarified in patients with NSCLC.
Therefore, we conducted this real-world retrospective study involving a relatively large sample size, to evaluate the efficacy of ICI-based treatment in advanced NSCLC patients who had previously been treated with ICIs and experienced disease progression, and to further if certain subgroups may benefit more from this approach. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-376/rc).
Methods
Study design and patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Hospital (No. 2020-905) and individual consent for this retrospective analysis was waived.
Patients with NSCLC who received ICI-based TBP with prior immunotherapy between January 2016 and July 2020 at the West China Hospital were reviewed. Patients’ clinical data, survival outcomes and follow-up information were collected for further analyses. Inclusion criteria were the following: pathologically confirmed primary NSCLC; stage IV according to 8th edition of the American Joint Committee on Cancer (AJCC) (25); confirmed disease progression following prior immunotherapy by the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1); patients received ICI-based treatment (anti-PD-1/PD-L1 inhibitor monotherapy or combined with other drugs) beyond progression with prior immunotherapy. Exclusion criteria included incomplete medical records, participation in clinical trials, and concurrent other malignancy meantime or in the past five years. Anonymized clinical information was collected from medical records, including sex, age, gene alteration status, PD-L1 expression status, histological subtype, smoking status, treatment history, Eastern Cooperative Oncology Group (ECOG) performance status at the start of second-round immunotherapy, and treatment outcomes. The ECOG score serves as an indicator of patients’ daily living physical status, ranging from 0 to 5 points.
Efficacy evaluation and statistical analysis
This study was designed to assess the efficacy of ICI-based TBP with prior immunotherapy based on the evaluation criteria RECIST 1.1. The PFS was defined as the time from the initiation of second-round immunotherapy to the date of confirmed disease progression or death from any cause and OS was defined as the time from the initiation of second-round immunotherapy to the date of death. The data were censored if the patient had not yet experienced progression or was still alive at the last follow-up. The follow-up data were obtained by telephone calls, outpatient records and inpatient records. The established deadline for follow-up was July 1, 2021. Objective response rate (ORR) was defined as the percentage of patient cases that achieved complete and partial responses (CR + PR), while the disease control rate (DCR) was defined as the percentage of patient cases that achieved stable disease, CR, or PR status (SD + CR + PR).
Survival curves were estimated by the Kaplan-Meier method, and the differences were compared by log-rank test. A Cox proportional hazards model was further applied to identify the factors which may influence clinical outcomes. The factors with statistical significance (P<0.05) in the univariate analysis and those clinically considered to be related to prognosis were further analyzed by multivariate analysis. A two-tailed P value was used and its less than 0.05 was regarded to be statistically significant. All statistical tests were analyzed using the computer software SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Clinicopathological characteristics
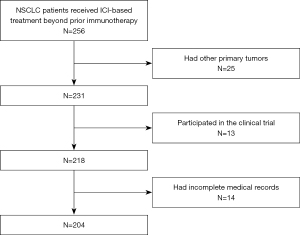
Between January 2016 and July 2020, 256 consecutive patients with NSCLC who received ICI-based TBP with prior immunotherapy at West China Hospital were enrolled. After screening, 25 were excluded due to other simultaneous primary tumors, and 13 patients were excluded because of participation in the clinical trial, 14 were excluded due to incomplete medical records. At the end, 204 patients were included in this study for analyses (Figure 1). All patients presented progression after the first round of ICI treatment, and that treatment approach gained a median progression-free survival of 5.3 (range, 4.6–6) months and an objective response rate of 37.3% (PR 76, CR 0). Regarding the progression pattern of prior immunotherapy, 50.5% (n=103) of the patients developed progression in the same initial lesions, while the rest 49.5% (n=101) of patients developed new metastatic lesions.
Clinical characteristics of patients at the time of second-round ICI treatment are presented in Table 1. The median age of the patients was 60 years (range, 33–85 years). Most patients were male (157/204, 77.0%), had adenocarcinoma (123/204, 60.3%), and presented an ECOG performance status of 0 or 1 (189/204, 92.6%). PD-L1 expression data were available for 152 patients (152/204, 74.5%). Negative PD-L1 expression was observed in 47 patients (tumor proportion score, TPS <1%), while weak positive PD-L1 expression was observed in 59 patients (TPS 1–49%) and strong positive PD-L1 expression was observed in 46 patients (TPS ≥50%). Molecular driver gene alterations were found in 69 patients, including epidermal growth factor receptor (EGFR; n=20), kirsten rat sarcoma viral oncogene homolog (KRAS; n=24), human epidermal growth factor receptor 2 (Her-2; n=11), v-raf murine sarcoma viral oncogene homolog B (BRAF, n=6), mesenchymal to epithelial transition factor (MET; n=4), ret proto-oncogene (RET; n=3), and anaplastic lymphoma kinase (ALK; n=1). Concerning the type of ICI drugs, 195 patients received anti-PD-1 agents (camrelizumab 22, nivolumab 36, pembrolizumab 73, tislelizumab 10, sintilimab 54), and only 9 patients received anti-PD-L1 agents (atezolizumab 4, durvalumab 5). As for the lines of the second-round ICI treatment, 45.1% (92/204) of patients received it as a second line, while 54.9% (112/204) of patients received it as a third or later line. Within which, 178 patients received the second-round ICI treatment without interval treatment, while 26 patients received cytotoxic chemotherapy-based regimens in the interval between two rounds of ICI treatment. ICI-based combination therapies were the major treatment strategy in the second-round ICI treatment (n=189; 92.6%), containing 63 patients combined with chemotherapy, 14 patients with chemotherapy plus radiotherapy, 3 patients with chemotherapy plus radiotherapy and antiangiogenic drugs, 21 patients with chemotherapy plus antiangiogenic drugs, 45 patients with radiotherapy, 15 patients with radiotherapy plus antiangiogenic drugs, and 28 patients with antiangiogenic drugs.
Table 1
| Clinical characteristic | N=204 |
|---|---|
| Age (years), median [range] | 60 [33–85] |
| ECOG, n (%) | |
| 0 | 101 (49.5) |
| 1 | 88 (43.1) |
| ≥2 | 15 (7.4) |
| Sex, n (%) | |
| Male | 157 (77.0) |
| Female | 47 (23.0) |
| Smoking status, n (%) | |
| Current/former smoker | 106 (52.0) |
| Never smoker | 98 (48.0) |
| Histologic type, n (%) | |
| Adenocarcinoma | 123 (60.3) |
| Squamous cell carcinoma | 64 (31.4) |
| Sarcomatoid carcinoma | 6 (2.9) |
| Lymphoepithelioma-like carcinoma | 5 (2.5) |
| Large cell carcinoma | 3 (1.5) |
| Adenoid cystic carcinoma | 2 (1.0) |
| Mucoepidermoid carcinoma | 1 (0.5) |
| PD-L1 expression, n (%) | |
| <1% | 47 (23.0) |
| 1–49% | 59 (28.9) |
| ≥50% | 46 (22.5) |
| Unknown | 52 (25.5) |
| Driver gene status, n (%) | |
| Negative | 135 (66.2) |
| Positive | 69 (33.8) |
| The line number of second round ICI treatment, n (%) | |
| 2 | 92 (45.1) |
| 3 | 68 (33.3) |
| ≥4 | 44 (21.6) |
| ICI treatment strategy, n (%) | |
| ICI monotherapy | 15 (7.4) |
| ICI-based combination therapy | 189 (92.6) |
| Other cancer treatments between two rounds of immunotherapy, n (%) | |
| Yes | 26 (12.7) |
| No | 178 (87.3) |
| Best response to prior immunotherapy, n (%) | |
| CR/PR | 76 (37.3) |
| SD/PD | 128 (62.7) |
| Progression-free survival of prior immunotherapy, n (%) | |
| ≥6 months | 110 (53.9) |
| <6 months | 94 (46.1) |
ICI, immune checkpoint inhibitor, ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Clinical outcomes
Data cutoff date was July 1, 2021. The median follow-up was 15.3 months (14.5–28.0 months) and the median number of cycles of treatment patients received was 7 (range, 1–32). At the end of follow-up, 174 patients encountered disease progression and 103 patients were deceased. The median PFS and OS under second-round ICI-based treatment were 5.0 months (95% CI: 4.5–5.5 months) and 15.7 months (95% CI: 14.7–16.8 months), respectively (Figure 2). The ORR was 9.3% (CR, n=1; PR, n=18) and DCR was 74.0% (CR + PR + SD, n=151).

To identify favorable factors related to treatment response, gene alteration status, smoking status, PD-L1 expression status, the line of ICI-based treatment beyond prior immunotherapy, the treatment efficacy and progression pattern of prior immunotherapy, immunotherapy treatment strategy, treatment insertion between two rounds of immunotherapy, and ECOG score were analyzed. The patients who responded well to prior immunotherapy achieved better survival outcomes than those who responded poorly: mPFS was 7.3 months for patients with complete or partial response (CR/PR) to prior ICI treatment versus 4.3 months for patients with stable or progressive disease (SD/PD) (P<0.0001), while mOS for these patients was 22.8 and 15.7 months, respectively (P<0.0001; Figure 3). Patients who achieved CR/PR/SD/PD as the best response to prior ICI treatment had an ORR of 100% (1/1), 6.7% (5/75), 10.1% (8/79), 10.2% (5/49) in the following second round of ICI treatment, respectively (Figure 4). The median PFS and OS were significantly better in patients who received ICI-based combination treatment compared to ICI monotherapy (mPFS 5.1 vs. 3.3 months, P=0.001; mOS 18.4 vs. 13.7 months, P=0.01; Figure 5). The clinical outcomes of patients without driver gene alterations were significantly better than those of patients with it (mPFS 5.5 vs. 3.5 months, P=0.001; mOS 16.1 vs. 13.9 months, P=0.012; Figure 6). In addition to the above results, according to the univariate analysis, higher PD-L1 expression (TPS ≥50%) and a better ECOG score (ECOG 0) were also correlated with improved PFS, while longer progression-free survival of prior immunotherapy (≥6 months) was related to better PFS and OS. The results of the multivariate analysis showed that ICI-based combination therapy [PFS: hazard ratio (HR), 0.48, 95% confidence interval (CI): 0.28–0.84, P=0.011] (OS: HR, 0.44, 95% CI: 0.23–0.85, P=0.014), lack of driver gene alterations (PFS: HR, 0.56, 95% CI: 0.40–0.79, P=0.001) (OS: HR, 0.57, 95% CI: 0.37–0.87, P=0.009), and good response (CR/PR) to prior immunotherapy(PFS: HR, 0.36, 95% CI:0.24–0.53, P<0.0001) (OS: HR, 0.31, 95% CI: 0.19–0.52, P<0.0001) were significantly associated with an improved PFS and OS (all P<0.05). In addition, disease progression involving new metastasis (OS: HR, 0.56, 95% CI: 0.37–0.84, P=0.005) was independently associated with favorable OS (P<0.05; Table 2).
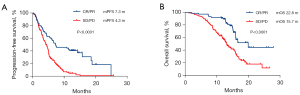
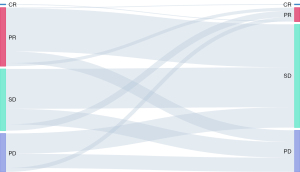

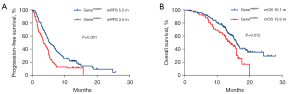
Table 2
| Risk factors | Univariable analysis | Multivariable analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS | OS | PFS | OS | ||||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||||
| Driver gene status (negative vs. positive) | 0.60 | 0.44–0.83 | 0.002 | 0.60 | 0.40–0.90 | 0.012 | 0.56 | 0.40–0.79 | 0.001 | 0.57 | 0.37–0.87 | 0.009 | |||
| Other treatments between two rounds of immunotherapy (no vs. yes) | 0.87 | 0.55–1.37 | 0.537 | 0.75 | 0.42–1.32 | 0.319 | 0.80 | 0.49–1.32 | 0.388 | 0.92 | 0.50–1.69 | 0.786 | |||
| Smoking status (yes vs. no) | 0.96 | 0.71–1.30 | 0.802 | 1.22 | 0.83–1.81 | 0.312 | 1.04 | 0.76–1.43 | 0.794 | 1.40 | 0.93–2.09 | 0.107 | |||
| PD-L1 expression (≥50% vs. <50%) | 0.61 | 0.41–0.90 | 0.013 | 0.72 | 0.43–1.19 | 0.196 | 0.84 | 0.55–1.29 | 0.420 | 1.02 | 0.59–1.76 | 0.957 | |||
| Best response to prior immunotherapy (CR/PR vs. SD/PD) | 0.38 | 0.27–0.54 | <0.0001 | 0.35 | 0.22–0.56 | <0.0001 | 0.36 | 0.24–0.53 | <0.0001 | 0.31 | 0.19–0.52 | <0.0001 | |||
| Progression-free survival of prior immunotherapy (≥6 vs. <6 months) | 0.72 | 0.53–0.98 | 0.035 | 0.58 | 0.39–0.86 | 0.007 | 1.17 | 0.83–1.66 | 0.371 | 0.78 | 0.51–1.20 | 0.255 | |||
| Progression pattern of prior immunotherapy (new metastasis vs. original lesions) | 1.05 | 0.78–1.41 | 0.763 | 0.72 | 0.49–1.06 | 0.096 | 0.90 | 0.66–1.24 | 0.533 | 0.56 | 0.37–0.84 | 0.005 | |||
| Second round ICI treatment lines (2 vs. ≥3) | 1.12 | 0.83–1.51 | 0.45 | 1.19 | 0.81–1.76 | 0.385 | 1.05 | 0.76–1.47 | 0.765 | 1.01 | 0.66–1.54 | 0.966 | |||
| Second round ICI treatment strategy (combination therapy vs. monotherapy) | 0.40 | 0.23–0.68 | 0.001 | 0.46 | 0.25–0.85 | 0.012 | 0.48 | 0.28–0.84 | 0.011 | 0.44 | 0.23–0.85 | 0.014 | |||
| ECOG score (0 vs. ≥1) | 0.84 | 0.72–0.97 | 0.021 | 0.83 | 0.68–1.01 | 0.064 | 0.78 | 0.56–1.10 | 0.157 | 0.85 | 0.57–1.29 | 0.446 | |||
ICI, immune checkpoint inhibitor; PD-L1, programmed death-ligand 1; ECOG, Eastern Cooperative Oncology Group; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
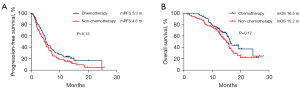
The subgroup analysis of different combined treatment modes demonstrated that patients who received the combination treatment modality including chemotherapy exhibited a more favorable trend in PFS and OS versus the chemotherapy-free cohort, although the difference was not statistically significant (mPFS 5.3 vs. 4.6 months, P=0.13; mOS 16.5 vs. 15.2 months, P=0.12; Figure 7).
Discussion
The introduction of ICIs has led to a major survival improvement among patients with advanced NSCLC (26), but the role of ICI-based treatment beyond prior immunotherapy remains debatable. Retrospective studies with a small sample size demonstrated a limited efficacy of receiving ICI monotherapy beyond progression after prior immunotherapy (17-24). The prospective study for NSCLC who benefited from prior immunotherapy reported better outcomes brought from ICI combined with targeted therapy beyond progression compared to ICI monotherapy, whereas treatment-related adverse events increased (15). We found a median PFS and OS of 5.0 months (95% CI: 4.5–5.5 months) and 15.7 months (95% CI: 14.7–16.8 months), respectively, in NSCLC patients who received ICI-based treatment after failure in previous immunotherapy, which was better than the data about NSCLC patients receiving salvage chemotherapy after the failure of immunotherapy (5-7). In further analyses, the combined treatment pattern, driver gene negative status, and good response to prior immunotherapy (CR/PR) were proven to be associated with better PFS and OS here.
Retrospective data concerning ICI retreatment following the recovery from the irAE (immune-related adverse event) suggested that survival benefit may occur in patients who had not well treatment response (SD) prior to irAE onset (25). But whether that association exists in the receipt of ICI retreatment after progression is in dispute. This study observed that patients who responded well to prior immunotherapy gained more survival benefits from the following ICI-based treatment. The same phenomenon has been observed in melanoma (27,28). Although the precise mechanism underlying this finding is unknown, a possible explanation may be that patients who respond well to prior immunotherapy develop immune memory cells (29,30), resulting in a quick reconstruction of the immune system when exposed to the next round of immunotherapy.
A synergistic anti-tumor effect of radiotherapy, chemotherapy, and antiangiogenic drugs with immunotherapy has been reported, and it leads to survival improvement (31-33). The current study demonstrated that ICI-based combination treatment therapy achieved higher efficacy over ICI monotherapy even in the context of ICI TBP after prior immunotherapy, and combination with chemotherapy is the main combination modality. Patients who received ICI combination therapy were further divided into two subgroups according to whether chemotherapy was administered, the chemotherapy-contained group experienced longer PFS and OS compared to the chemotherapy-free group. Because of the retrospective nature of this study, it was not possible to further analyze which combination of chemotherapy regimens is more conducive to survival benefits. The intervening chemotherapy administration between two rounds of ICI treatment may sensitize the follow-up ICI treatment according to a real-world study experience focusing on immunotherapy re-challenge (30). But another retrospective study reported that not receiving systemic treatment between the two rounds of ICI treatment was a favorable factor related to better clinical outcomes (34). Our study found that systemic treatment in the interval between the two rounds of immunotherapy was not associated with clinical treatment outcome.
Previous clinical studies have demonstrated that the efficacy of immunotherapy among advanced NSCLC patients with positive driver gene alterations is limited due to an unfavorable microenvironment (35-37). The present study provided evidence that driver gene positive status is also a negative predictor of the efficacy of ICI-based treatment beyond prior immunotherapy. Although PD-L1 expression has been regarded as a stable biomarker to identify patients with advanced NSCLC who benefit from anti-PD-1/PD-L1 therapy (38,39), in the present study, such a relationship was lacking in the context of receiving second-round immunotherapy after the failure of prior immunotherapy.
This study has some limitations. Firstly, more analysis could not be carried out limited by the sample size and retrospective nature of the study. Secondly, information relating to PD-L1 expression was gained at the initial diagnosis. The data on PD-L1 expression before the second round of immunotherapy is lacking. Thirdly, selection bias exists, as subsequent treatment timing and regimes were mainly determined by the attending doctor.
Conclusions
Patients with advanced NSCLC may benefit from ICI-based treatment beyond prior immunotherapy. In addition, ICI-based combination therapy, lack of targetable gene alterations, and good response to prior immunotherapy were found to be important factors associated with survival benefits. Large prospective clinical trials are needed to further confirm these findings.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-376/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-376/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-376/coif). TTS reports that he provides strategic and scientific recommendations as a member of the Advisory Board and speaker for Novocure, Inc., and also as a member of the Advisory Board to Galera Therapeutics, which are not in any way associated with the content or disease site as presented in this manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Hospital (No. 2020-905) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Doroshow DB, Sanmamed MF, Hastings K, et al. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin Cancer Res 2019;25:4592-602. [Crossref] [PubMed]
- Horvath L, Thienpont B, Zhao L, et al. Overcoming immunotherapy resistance in non-small cell lung cancer (NSCLC) - novel approaches and future outlook. Mol Cancer 2020;19:141. [Crossref] [PubMed]
- Bersanelli M, Buti S, Giannarelli D, et al. Chemotherapy in non-small cell lung cancer patients after prior immunotherapy: The multicenter retrospective CLARITY study. Lung Cancer 2020;150:123-31. [Crossref] [PubMed]
- Costantini A, Corny J, Fallet V, et al. Efficacy of next treatment received after nivolumab progression in patients with advanced nonsmall cell lung cancer. ERJ Open Res 2018;4:e00120-2017. [Crossref] [PubMed]
- Metro G, Addeo A, Signorelli D, et al. Outcomes from salvage chemotherapy or pembrolizumab beyond progression with or without local ablative therapies for advanced non-small cell lung cancers with PD-L1 ≥50% who progress on first-line immunotherapy: real-world data from a European cohort. J Thorac Dis 2019;11:4972-81. [Crossref] [PubMed]
- Waterhouse DM, Garon EB, Chandler J, et al. Continuous Versus 1-Year Fixed-Duration Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: CheckMate 153. J Clin Oncol 2020;38:3863-73. [Crossref] [PubMed]
- Herbst RS, Garon EB, Kim DW, et al. Long-term follow-up in the Keynote-010 study of pembrolizumab (pembro) for advanced NSCLC, including in patients (pts) who completed 2 years of pembro and pts who received a second course of pembro. Ann Oncol 2018;29:X42-3. [Crossref]
- Ricciuti B, Genova C, Bassanelli M, et al. Safety and Efficacy of Nivolumab in Patients With Advanced Non-small-cell Lung Cancer Treated Beyond Progression. Clin Lung Cancer 2019;20:178-185.e2. [Crossref] [PubMed]
- Stinchcombe TE, Miksad RA, Gossai A, et al. Real-World Outcomes for Advanced Non-Small Cell Lung Cancer Patients Treated With a PD-L1 Inhibitor Beyond Progression. Clin Lung Cancer 2020;21:389-394.e3. [Crossref] [PubMed]
- Ge X, Zhang Z, Zhang S, et al. Immunotherapy beyond progression in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res 2020;9:2391-400. [Crossref] [PubMed]
- Teraoka S, Akamatsu H, Takamori S, et al. A phase II study of nivolumab rechallenge therapy in advanced NSCLC patients who responded to prior anti-PD-1/L1 inhibitors: West Japan Oncology Group 9616L. Ann Oncol 2021;32:S1001-1002. [Crossref]
- Puri S, Tanvetyanon T, Creelan B, et al. Phase II Study of Nivolumab and Ipilimumab Combined With Nintedanib in Recurrent Non-Small Cell Lung Cancer. J Thorac Oncol 2021;16:S924-S925. [Crossref]
- Lee J, Park S, Jung HA, et al. Combination of Bevacizumab + Atezolizumab (A) Who Progressed On A In Pretreated NSCLC Patients: An Open-Label, Two-Stage, Phase II Trial. J Thorac Oncol 2021;16:S925. [Crossref]
- Leal TA, Berz D, Rybkin I, et al. MRTX-500: Phase II trial of sitravatinib (sitra) plus nivolumab (nivo) in patients (pts) with non-squamous (NSQ) non-small cell lung cancer (NSCLC) progressing on or after prior checkpoint inhibitor (CPI) therapy. Ann Oncol 2021;32:S949. [Crossref]
- Katayama Y, Shimamoto T, Yamada T, et al. Retrospective Efficacy Analysis of Immune Checkpoint Inhibitor Rechallenge in Patients with Non-Small Cell Lung Cancer. J Clin Med 2019;9:102. [Crossref] [PubMed]
- Kitagawa S, Hakozaki T, Kitadai R, et al. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: Case series and literature review. Thorac Cancer 2020;11:1927-33. [Crossref] [PubMed]
- Fujita K, Yamamoto Y, Kanai O, et al. Retreatment with anti-PD-1 antibody in non-small cell lung cancer patients previously treated with anti-PD-L1 antibody. Thorac Cancer 2020;11:15-8. [Crossref] [PubMed]
- Fujita K, Uchida N, Yamamoto Y, et al. Retreatment With Anti-PD-L1 Antibody in Advanced Non-small Cell Lung Cancer Previously Treated With Anti-PD-1 Antibodies. Anticancer Res 2019;39:3917-21. [Crossref] [PubMed]
- Niki M, Nakaya A, Kurata T, et al. Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget 2018;9:32298-304. [Crossref] [PubMed]
- Watanabe H, Kubo T, Ninomiya K, et al. The effect and safety of immune checkpoint inhibitor rechallenge in non-small cell lung cancer. Jpn J Clin Oncol 2019;49:762-5. [Crossref] [PubMed]
- Fujita K, Uchida N, Kanai O, et al. Retreatment with pembrolizumab in advanced non-small cell lung cancer patients previously treated with nivolumab: emerging reports of 12 cases. Cancer Chemother Pharmacol 2018;81:1105-9. [Crossref] [PubMed]
- Reinhorn D, Jacobi O, Icht O, et al. Treatment beyond progression with immune checkpoint inhibitors in non-small-cell lung cancer. Immunotherapy 2020;12:235-43. [Crossref] [PubMed]
- Wankhede D. Evaluation of Eighth AJCC TNM Sage for Lung Cancer NSCLC: A Meta-analysis. Ann Surg Oncol 2021;28:142-7. [Crossref] [PubMed]
- Brozos-Vázquez EM, Díaz-Peña R, García-González J, et al. Immunotherapy in nonsmall-cell lung cancer: current status and future prospects for liquid biopsy. Cancer Immunol Immunother 2021;70:1177-88. [Crossref] [PubMed]
- Nomura M, Otsuka A, Kondo T, et al. Efficacy and safety of retreatment with nivolumab in metastatic melanoma patients previously treated with nivolumab. Cancer Chemother Pharmacol 2017;80:999-1004. [Crossref] [PubMed]
- Betof Warner A, Palmer JS, Shoushtari AN, et al. Long-Term Outcomes and Responses to Retreatment in Patients With Melanoma Treated With PD-1 Blockade. J Clin Oncol 2020;38:1655-63. [Crossref] [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Giaj Levra M, Cotté FE, Corre R, et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: A national data base analysis. Lung Cancer 2020;140:99-106. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 2016;13:516-24. [Crossref] [PubMed]
- Halmos B, Burke T, Kalyvas C, et al. Pembrolizumab+chemotherapy versus atezolizumab+chemotherapy+/-bevacizumab for the first-line treatment of non-squamous NSCLC: A matching-adjusted indirect comparison. Lung Cancer 2021;155:175-82. [Crossref] [PubMed]
- Gobbini E, Toffart AC, Pérol M, et al. Immune Checkpoint Inhibitors Rechallenge Efficacy in Non-Small-Cell Lung Cancer Patients. Clin Lung Cancer 2020;21:e497-510. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Berghoff AS, Bellosillo B, Caux C, et al. Immune checkpoint inhibitor treatment in patients with oncogene- addicted non-small cell lung cancer (NSCLC): summary of a multidisciplinary round-table discussion. ESMO Open 2019;4:e000498. [Crossref] [PubMed]
- Guisier F, Dubos-Arvis C, Viñas F, et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients With Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J Thorac Oncol 2020;15:628-36. [Crossref] [PubMed]
- Prelaj A, Tay R, Ferrara R, et al. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer 2019;106:144-59. [Crossref] [PubMed]
- Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020;126:260-70. [Crossref] [PubMed]


