
NRF2-pathway mutations predict radioresistance in non-small cell lung cancer
Approximately 54% of non-small cell lung cancer (NSCLC) patients present with early-stage or locally-advanced disease and are potentially curable, of which 40% receive radiotherapy as a part of their initial course of treatment (1,2). Although biomarkers routinely guide treatment decisions for systemic therapies in NSCLC, there are no clinical biomarkers that predict response to radiotherapy. Two independent studies have recently converged on alterations in the NRF2 pathway as potential biomarkers of radioresistance in NSCLC (3,4).
In Clinical Cancer Research, Sitthideatphaiboon et al. identify STK11 (LKB1) mutations as a predictive biomarker of radioresistance in NSCLC (3). The authors retrospectively analyze a cohort of 194 stage I–III patients treated with radiotherapy and find STK11 mutations to be the strongest predictor of disease-free survival (DFS) and overall survival (OS). They demonstrate that STK11 and KEAP1 mutations confer radioresistance in NSCLC xenograft models and identify glutaminase as a potential therapeutic target to overcome NRF2-mediated radioresistance in vitro. This manuscript complements a recent publication in Cancer Discovery, which identifies pathogenic KEAP1/NFE2L2 (NRF2) mutations as a biomarker of radioresistance in NSCLC, which can similarly be reversed by glutaminase inhibition in vitro (4). Together, these studies support a unifying theory for NRF2-mediated radioresistance and “glutamine-addiction” in NSCLC (5,6).
The study by Sitthideatphaiboon et al. (3) is the first to examine clinical outcomes based on STK11 status in a radiotherapy cohort. The authors acknowledge limitations, including its retrospective nature, limited sample size, and inability to access KEAP1 mutations. We, therefore, sought to validate their findings using three large prospective cohorts (Table 1). Putative driver (pathogenic) mutations versus variants of unknown significance were defined via OncoKB and Cancer Hotspots annotations in the cBioportal (https://www.cbioportal.org/). Among non-metastatic NSCLC patients in The Cancer Genome Atlas (TCGA) (n=736, 34% prospective) (7), STK11 pathogenic mutations were associated with poorer OS in patients who received radiotherapy {n=83, HR: 3.03 [95% confidence interval (CI): 1.38–6.68]}, but not in patients who received no radiotherapy (n=547). Including KEAP1 and NFE2L2 pathogenic mutations in adenocarcinomas (8) increased the number of patients with potentially radioresistant tumors from 6.9% to 9.8% and was a stronger predictive biomarker. KEAP1/NFE2L2/STK11 mutations were associated with poorer OS [HR: 3.78 (1.85–7.74)], progression-free survival [3.80 (1.87–7.72)], DFS [4.46 (0.94–21.1)], and disease-specific survival [4.83 (2.20–10.6)] only in patients that received radiotherapy (interaction with treatment P=0.02, 0.09, 0.23, and 0.02, respectively; Table 1; Figure 1). Additionally, KEAP1/NFE2L2/STK11 mutations were not prognostic in patients that received R0 resections (n=592), but were associated with poorer OS [HR: 2.37 (1.00–5.63)] and DFS [3.11 (1.37–7.05)] in patients with less than R0 or no resection (n=137), suggesting that KEAP1/NFE2L2/STK11 status is prognostic in patients who are assumed to undergo adjuvant or definitive radiotherapy. These findings were confirmed in multivariable analysis and after stratifying by stage. Consistent with these findings, in MSK-IMPACT (n=961) (9), KEAP1/NFE2L2/STK11 mutations were associated with poorer OS in tumors that were biopsied [n=407; HR: 2.04 (1.36–3.04)] but not resected (n=378). Similarly, in the TRACERx study (n=100) (10), KEAP1/NFE2L2/STK11 status was not significantly prognostic in patients who underwent curative resection. However, when stratifying by lymph node status, KEAP1/NFE2L2/STK11 status was prognostic of recurrence or death [HR: 5.82 (1.36–24.4)] only in patients with positive lymph nodes after surgery (n=24).
Table 1
| Treatment subgroup | N | STK11 | KEAP1*/NFE2L2*/STK11 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | Interaction | HR | 95% CI | P value | Interaction | |||
| TCGA Pan-Cancer1 | 736 | 6.9% | 9.8% | |||||||
| Overall survival | 0.09 | 0.02# | ||||||||
| No radiotherapy | 547 | 1.39 | 0.81, 2.39 | 0.24 | 1.44 | 0.92, 2.24 | 0.11 | |||
| Radiotherapy | 83 | 3.03 | 1.38, 6.68 | 0.006# | 3.78 | 1.85, 7.74 | <0.001# | |||
| Progression-free survival | 0.52 | 0.09 | ||||||||
| No radiotherapy | 2.30 | 1.41, 3.75 | <0.001# | 1.87 | 1.21, 2.90 | 0.005# | ||||
| Radiotherapy | 3.14 | 1.44, 6.86 | 0.004# | 3.80 | 1.87, 7.72 | <0.001# | ||||
| Disease-free survival | 0.36 | 0.23 | ||||||||
| No radiotherapy | 1.89 | 0.92, 3.90 | 0.09 | 1.54 | 0.82, 2.89 | 0.18 | ||||
| Radiotherapy | 4.46 | 0.94, 21.1 | 0.06 | 4.46 | 0.94, 21.1 | 0.06 | ||||
| Disease-specific survival | 0.20 | 0.02# | ||||||||
| No radiotherapy | 1.89 | 0.95, 3.76 | 0.07 | 1.62 | 0.88, 2.97 | 0.12 | ||||
| Radiotherapy | 3.54 | 1.49, 8.37 | 0.004# | 4.83 | 2.20, 10.6 | <0.001# | ||||
| TCGA Firehose2 | 767 | 4.2% | 5.3% | |||||||
| Overall survival | 0.21 | 0.08 | ||||||||
| R0 resection | 592 | 1.07 | 0.59, 1.97 | 0.82 | 1.04 | 0.62, 1.76 | 0.88 | |||
| Less than R0 | 137 | 2.04 | 0.80, 5.20 | 0.13 | 2.37 | 1.00, 5.63 | 0.05# | |||
| Disease-free survival | 0.27 | 0.08 | ||||||||
| R0 resection | 1.47 | 0.80, 2.71 | 0.22 | 1.32 | 0.76, 2.28 | 0.32 | ||||
| Less than R0 | 2.64 | 1.11, 6.31 | 0.03# | 3.11 | 1.37, 7.05 | 0.007# | ||||
| MSK-IMPACT3 | 961 | 13.7% | 16% | |||||||
| Overall survival | 0.79 | 0.45 | ||||||||
| Resection | 378 | 1.96 | 1.02, 3.78 | 0.05# | 1.48 | 0.77, 2.86 | 0.24 | |||
| Biopsy | 407 | 1.77 | 1.15, 2.73 | 0.009# | 2.04 | 1.36, 3.04 | <0.001# | |||
| TRACERx4 | 100 | 7% | 13% | |||||||
| Relapse-free survival | 0.36 | 0.26 | ||||||||
| Resection, N0 | 74 | 1.01 | 0.13, 7.75 | >0.99 | 1.70 | 0.47, 6.11 | 0.41 | |||
| Resection, N+ | 24 | 2.27 | 0.62, 8.26 | 0.21 | 5.82 | 1.36, 24.4 | 0.02# | |||
*, pathogenic KEAP1/NFE2L2 mutations in adenocarcinomas; 1, stage I, II, and III represent 52%, 30%, and 18% of patients, respectively; 2, stage I, II, and III represent 44%, 25%, and 15% of patients, respectively; 3, stage not provided; 4, stage I, II, and III represent 62%, 24%, and 14% of patients, respectively; #, statistically significant. TCGA, The Cancer Genome Atlas; N0/+, lymph node negative/positive; HR, hazard ratio; CI, confidence interval.
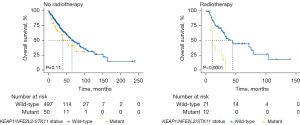
Here, we provide further evidence that KEAP1/NFE2L2/STK11 mutations are common and predictive of outcomes after radiotherapy in the TCGA database. We additionally show in three large prospective cohorts with almost 1,800 patients that KEAP1/NFE2L2/STK11 mutations are not prognostic in patients who undergo curative surgery. Collectively, these data support further testing of KEAP1/NFE2L2/STK11 as a biomarker of radioresistance and to identify patients that may be eligible for clinical trials targeting the NRF2 pathway. Limitations of our study include retrospective analysis of datasets with limited clinical information available. Prospective validation of these findings is warranted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-292/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-292/coif). LLC reports stocks or stock options from Kojin Therapeutics. RCS reports personal fees and non-financial support from Maze Therapeutics. SKC reports personal fees and non-financial support from AbbVie, Sanofi. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ganti AK, Klein AB, Cotarla I, et al. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non-Small Cell Lung Cancer in the US. JAMA Oncol 2021;7:1824-32. [Crossref] [PubMed]
- Kinslow CJ, May MS, Saqi A, et al. Large-Cell Neuroendocrine Carcinoma of the Lung: A Population-Based Study. Clin Lung Cancer 2020;21:e99-e113. [Crossref] [PubMed]
- Sitthideatphaiboon P, Galan-Cobo A, Negrao MV, et al. STK11/LKB1 Mutations in NSCLC Are Associated with KEAP1/NRF2-Dependent Radiotherapy Resistance Targetable by Glutaminase Inhibition. Clin Cancer Res 2021;27:1720-33. [Crossref] [PubMed]
- Binkley MS, Jeon YJ, Nesselbush M, et al. KEAP1/NFE2L2 Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition. Cancer Discov 2020;10:1826-41. [Crossref] [PubMed]
- Romero R, Sayin VI, Davidson SM, et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med 2017;23:1362-8. [Crossref] [PubMed]
- Galan-Cobo A, Sitthideatphaiboon P, Qu X, et al. LKB1 and KEAP1/NRF2 Pathways Cooperatively Promote Metabolic Reprogramming with Enhanced Glutamine Dependence in KRAS-Mutant Lung Adenocarcinoma. Cancer Res 2019;79:3251-67. [Crossref] [PubMed]
- Liu J, Lichtenberg T, Hoadley KA, et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018;173:400-16.e11. [Crossref] [PubMed]
- Goeman F, De Nicola F, Scalera S, et al. Mutations in the KEAP1-NFE2L2 Pathway Define a Molecular Subset of Rapidly Progressing Lung Adenocarcinoma. J Thorac Oncol 2019;14:1924-34. [Crossref] [PubMed]
- Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23:703-13. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2109-21. [Crossref] [PubMed]


