
Proteomic analysis of plasma exosomes in patients with non-small cell lung cancer
Introduction
Lung cancer has been the most commonly diagnosed cancer for the last several decades (1). About 15% are small cell lung cancer and 85% of lung cancers are non-small cell lung cancer (NSCLC). The following NSCLC subtypes can be distinguished histologically: large cell carcinoma (3%), squamous cell carcinoma (20%) and adenocarcinoma and (38.5% of all lung cancers) (2). The treatment of NSCLC depends on stage and the clinical performance of the patients. Surgery is usually recommended for early-stage patients. Although significant progress has been made in the diagnosis and treatment of lung cancer, patient prognosis remains unsatisfactory (3,4) and the overall survival rate of lung cancer patients being disappointingly low. There may be several factors contributing to this low overall survival rate, such as the lack of effective screening strategies to detect early tumors, the obvious drug resistance of lung cancer cells, and the insufficient understanding of the multifactor cell network activated or inhibited during the pathogenesis of cancer (5). Furthermore, the identification of a reliable biomarker to predict tumor response to treatments and patient prognosis remains elusive (6). Over the past 20 years, various proteomics approaches have been developed, including mass spectrometry (MS) (7). Used with the high throughput technique and powerful statistical software, a large number of peptides and proteins are able to be identified and quantified with high efficiency. MS-based proteomics has gained much traction in the last decade (8), with the potential to identify relevant biomarkers that can be accurately quantified in multiplex assays (9). Indeed, proteomics may contribute significantly to the understanding of the mechanisms involved in NSCLC progression and response to treatment (10).
Exosomes are small, double-layered lipid membrane vesicles, initially formed by the endocytic process. They are nanoscale vesicles, ranging from 30–100 nm in diameter, that contain bioactive molecules such as proteins, DNA, mRNAs, and micro RNAs (miRNAs) (11). Most cell types secrete exosomes, in particular tumor cells. Tumor-derived exosomes play an important role in the communication between tumor cells and their microenvironment, contributing a favorable environment for tumor progression (12). The previous studies indicated that exosomes can promote tumorigenesis, invasion, migration through the transfer of the mRNAs, miRNAs and proteins in NSCLC (13-15). Besides, exosomes can mediate the microenvironment communication, modulating the immune response and aiding the metastasis, which will influence the tumorigenesis (15). Tumor-derived exosomes also promote the migration through influencing the integrity of vascular barriers (16). The exosomes from different cells have different functions. Mesenchymal stem cells derived exosomes induced the epithelial-mesenchymal transition and the infiltration of inflammatory cells in cancer, which can promote the growth of the NSCLC (17). M2 macrophage derived exosomes can enhance the aerobic glycolysis and inhibit the apoptosis of the tumor (18). It is noteworthy that exosomes have also been isolated in most biological fluids, including plasma, pleural effusions and bronchial lavage (19). Moreover, exosomes provide a protective vesicle for transporting small RNAs against degradation by RNases (20). These special features make exosomes an ideal specimen for liquid biopsy (21,22). Sandfeld-Paulsen et al. (23) explored the potential of exosomes as diagnostic biomarkers in NSCLC adenocarcinoma and squamous cell carcinoma and found that the markers CD151, CD171, and tetraspanin 8 were highly differentially expressed in patients with cancers of all histological subtypes compared to patients without cancer. The purpose of the study is to examine the expression of exosome proteins in NSCLC adenocarcinoma and squamous cell carcinoma at different stages, identifying potential prognostic biomarkers for clinical diagnosis. We present the following article in accordance with the MDAR reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-467/rc).
Methods
Patients
This study was a prospective study without interventions to perform the comprehensive exosome protein profile of NSCLC. Between December 2019 to April 2021, all patients with “pulmonary mass who underwent “lobectomy” at the Shanghai Pulmonary Hospital were candidates for this study. Lung tissue samples were collected within 30 minutes after surgical resection and quick-frozen in liquid nitrogen, then transferred to −80 ℃ within 30 minutes. Clinical data (including sex, age at diagnosis, smoking history), was retrieved from an electronic medical database. In addition, tumor volume and pathological type were recorded, and histopathology of all cases was confirmed by at least 2 pathologists based on high-resolution images of hematoxylin and eosin (H&E) sections. Whole blood samples were collected preoperatively for subsequent multiple proteomics analyses. After excluding patients with pulmonary tumors other than NSCLC, or patients with no informed consent, plasma exosomes were extracted from whole blood sample. The Ethics Committee of the Shanghai Pulmonary Hospital (No. K20-197Y) approved the study protocol, and all patients provided written informed consent. The institutional review boards at Shanghai Pulmonary Hospital reviewed all protocols. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All consent documentation adhered to the Clinical Proteomic Tumor Analysis Consortium (CPTAC) guidelines.
Extraction of plasma exosomes
Blood samples were extracted from patients using blood collection tubes with sodium citrate anticoagulant and centrifuged at 2,500 g for 10 minutes at room temperature. Plasma was transferred to a new tube and was centrifuged at 15,000 g for 10 minutes at 4 ℃ in order to remove the platelets. The exosomes were harvested after a final centrifugation of 100,000 g for 2 hours and resuspended in 100 µL phosphate buffered saline (PBS).
Extraction of protein
Exosomes were lysed with moderate lysis buffer containing protease inhibitors, using an ultrasonic processor and placed on ice for 30 minutes. The lysate was centrifuged at 12,000 g for 20 minutes at 4 ℃ and the supernatant was collected. The BCA assay (Yeason, Shanghai, China) was used to detect the protein concentration.
Trypsin digestion and peptide desalination
The extracted protein samples underwent reductive alkylation and digestion with trypsin (diluted with ammonium bicarbonate) at 37 ℃ overnight. On the following day, the peptides were obtained after centrifugation and dried by centrifugal concentration.
The peptides were desalted using a Zip tip C18 column (75 µm × 20 cm, particle size 1.9 µm) at a flow rate of 200 nl/min and analyzed by Orbitrap Fusion Lumos MS (Thermo Fisher Scientific) interfaced with Easy-nLC 1200 nanoflow liquid chromatography system.
LC-MS/MS for proteomics analyses
Mass spectra were obtained in a data-dependent manner and full scans (MS1) were obtained at a resolution of 60,000 and a mass range of 375 to 1,600 m/z. The ten most intense precursor ions were chosen for MS/MS and fragmented using higher-energy collisional dissociation (HCD) at a collision energy of 30. A label-free quantification tool, which is implemented in the MaxQuant software, was used to acquire normalized label-free quantification intensity of proteins.
The Uniprot_Human database was used to search the obtained MS data in the MaxQuant (v1.5.8.3) environment. The search parameters were set as follows: trypsin as the protease; oxidation (M), acetyl (protein N-term), and carbamidomethyl I as modifications; up to 2 missed cleavages; minimal peptide length set as 7; mass tolerance for MS2 was 20 ppm. A stringent false discovery rate (FDR) <0.01 was used to filter PSM and a FDR <0.05 was used to filter protein identifications.
Functional enrichment analysis of differently expressed proteins
To further understanding the functional annotation of the differently expressed proteins, GO analysis and KEGG analysis of the differentially expressed miRNAs was performed using the plug-in of ClueGO Cytoscape 3.8.2 software. An adjusted false discovery rate (FDR) P<0.05 was considered statistically significant. The top 10 significantly GO terms in biological processes (BPs); molecular functions (MFs); cellular components (CCs) and the top 10 KEGG pathways were selected.
Statistical analysis
The SPSS software (Statistical Package for the Social Sciences, Chicago, IL, USA) version 26.0, RStudio V1.2.1335 (RStudio Inc., Boston, USA) and GraphPad Prism (San Diego, CA, USA) version 9.0 was used to conduct statistical analyses. We used the Chi-square tests to analyze categorical data and the Student’s t-test or Mann-Whitney U test was used to analyze continuous data. Continuous variables are shown as mean ± standard deviation (SD) or medians (interquartile range) and categorical variables are showed as percentage numbers (%). P<0.05 was considered statistically significant.
Results
The baseline characteristics of patients with NSCLC
A total of 230 patients had lobectomy for pulmonary tumor between December 2019 and April 2021 at the Shanghai Pulmonary Hospital (Figure 1). Among of them, 195 NSCLC tumors were diagnosed pathologically. Finally, the exosome protein profile was performed for 26 plasma samples from NSCLC patients (including 14 males and 12 females, aged 47–79 years) which were obtained before surgery from patients after excluding patients with no informed consent or too small tumor volume, preoperative neoadjuvant therapy. Table 1 summarizes the baseline characteristics of the patients. A total of 8 patients presented with lung squamous cell carcinoma and 18 presented with lung adenocarcinoma. Genetic mutations were present in 12 patients, including 3 patients with epidermal growth factor receptor (EGFR) exon 19 deletion (E19del) mutations, 7 patients with an amino acid substitution at codon 858 in exon 21 (L858R) mutations, and 2 patients with echinoderm microtubule-associated protein-like 4 gene-ALK (EML4-ALK) mutations.
Table 1
| Characteristics | NSCLC patients (N=26) |
|---|---|
| Age (years), mean ± SD | 64.85±8.84 |
| Sex, n (%) | |
| Male | 14 (53.85) |
| Female | 12 (46.15) |
| Lesion site | |
| Left lung | 8 |
| Right lung | 18 |
| Pathological pattern | |
| Squamous cell carcinoma | 8 |
| Adenocarcinoma | 18 |
| Pathological stage | |
| I/II/III/IV | 8/13/5/0 |
| Lymph node metastasis, n (%) | 13 (50.0) |
| Genetic mutations, n (%) | 12 (46.15) |
| E19del mutations | 3 (11.54) |
| L8585R mutations | 7 (26.92) |
| EML4-ALK mutations | 2 (7.69) |
| Smoking history, n (%) | |
| Never | 21 (80.77) |
| Former | 0 |
| Current smoking status | 5 (19.23) |
NSCLC, non-small cell lung cancer; E19del mutations, epidermal growth factor receptor (EGFR) exon 19 deletion mutations; L858R mutations, an amino acid substitution at codon 858 in exon 21 mutations; EML4-ALK mutations, echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) mutations.
The characteristics of the differentially expressed proteins derived from plasma in NSCLC patients
The top 20 most abundantly expressed proteins in the plasma-derived exosomes of NSCLC patients is shown in Table 2. The most abundantly expressed proteins in plasma-derived exosomes in NSCLC patients are apolipoprotein B (APOB), immunoglobulin heavy constant mu (IGHM), and albumin (ALB).
Table 2
| Total | Lung squamous cell carcinoma | Lung adenocarcinoma |
|---|---|---|
| APOB | APOB | APOB |
| IGHM | IGHM | IGHM |
| ALB | ALB | ALB |
| APOE | APOC3 | APOE |
| APOC3 | APOE | KRT10 |
| KRT10 | LPA | APOC3 |
| KRT1 | APOC2 | KRT1 |
| APOA1 | KRT1 | LPA |
| APOC2 | KRT10 | FGA |
| FGA | APOA1 | APOA1 |
| C4BPA | C4BPA | C4BPA |
| FGB | HP | FGB |
| HP | FGA | APOC2 |
| IGLC2 | FGB | IGLC2 |
| IGKC | KRT2 | IGKC |
| IGHG2 | KRT9 | HP |
| FGG | VWF | IGHG2 |
| KRT2 | IGLC2 | FGG |
| KRT9 | FGG | CD5L |
| CYP2R1 | IGKC | KRT9 |
APOB, apolipoprotein B-100; IGHM, immunoglobulin heavy constant mu; ALB, albumin; APOE, apolipoprotein E; APOC3, apolipoprotein C-III; KRT10, type I cytoskeletal 10; KRT1, type II cytoskeletal 1; APOA1, apolipoprotein A-I; APOC2, apolipoprotein C-II; FGA, fibrinogen alpha chain; C4BPA, c4b-binding protein alpha chain; FGB, fibrinogen beta chain; HP, haptoglobin; IGLC2, immunoglobulin lambda constant 2; IGKC, immunoglobulin kappa constant; IGHG2, immunoglobulin heavy constant gamma 2; FGG, fibrinogen gamma chain; KRT2, type II cytoskeletal 2 epidermal; KRT9, type I cytoskeletal 9; vitamin D 25-hydroxylase; VWF, von Willebrand factor; CD5L, CD5 antigen-like.
The characteristics of the differentially expressed proteins derived from plasma in lung squamous cell carcinoma and lung adenocarcinoma
The top 20 most abundantly expressed proteins derived from the plasma-derived exosomes of patients with lung squamous cell carcinoma and patients with lung adenocarcinoma were almost identical, with the exception of VWF in lung squamous cell carcinoma and CD5L in lung adenocarcinoma (Table 2).
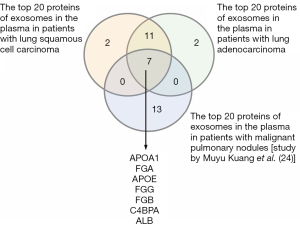
The overlapping plasma-derived exosome proteins in our study and a published dataset
Among the top 20 abundant proteins in lung squamous cell carcinoma and lung adenocarcinoma, there were 7 overlapping proteins between our study and a similar study by Kuang et al. (24) (Figure 2). These 7 proteins were apolipoprotein A-I (APOA1), fibrinogen alpha chain (FGA), apolipoprotein E (APOE), fibrinogen gamma chain (FGG), fibrinogen beta chain (FGB), C4b-binding protein alpha chain (C4BPA), and albumin (ALB) (24).
GO and KEGG analyses
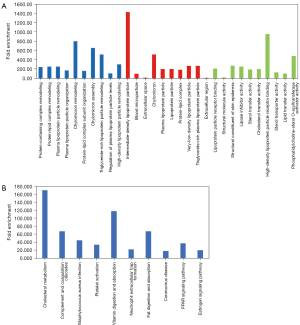
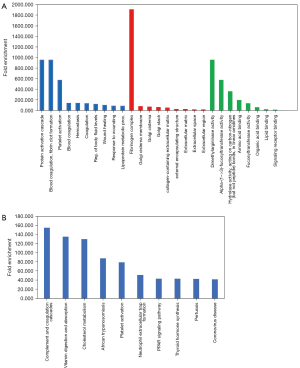
GO and KEGG analyses were performed on all 26 NSCLC patients with squamous carcinoma and adenocarcinoma, in order to understand the function of the differentially expressed genes. For the 26 NSCLC patients, GO analysis showed that the top 20 most abundant plasma-derived proteins were mainly associated with (Figure 3A): (I) the BPs chylomicron remodeling, chylomicron assembly, and triglyceride-rich lipoprotein particle remodeling; (II) the CCs intermediate density lipoprotein particle, chylomicron, and very low density lipoprotein particles; and (III) molecular functions involved in high-density lipoprotein particle receptor binding, phosphatidylcholine-sterol O-acyltransferase activator activity, and structural constituents of skin epidermis. As shown in Figure 3B, KEGG analysis revealed that the top 20 most abundant plasma-derived proteins were mainly enriched in cholesterol metabolism, vitamin digestion and absorption, and fat digestion and absorption.
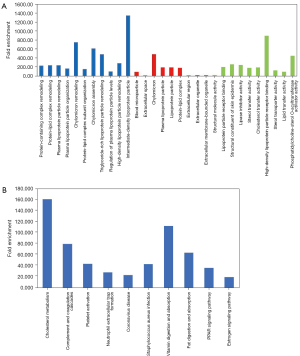
Figures 4,5 highlight the enriched GO and KEGG pathways for squamous-cell carcinoma and adenocarcinoma. The top 5 enriched GO terms of the differentially expressed proteins in lung squamous carcinoma and lung adenocarcinoma were highly similar. Among them, the most enriched pathway was the cholesterol metabolism pathway.
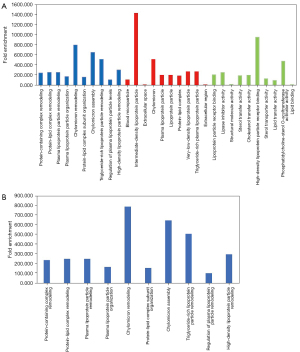
As shown in Figure 6A, the 7 overlapping exosome proteins in our study and the report by Kuang et al. (24). were associated with the BPs including protein activation cascade, blood coagulation, fibrin clot formation, platelet activation; the CCs fibrinogen complex, golgi cisterna membrane, and golgi cisterna; and the MFs dimethylargininase activity, alpha-(1->3)-fucosyltransferase activity, and amino acid binding. KEGG analysis showed that these proteins were enriched in complement and coagulation cascades, vitamin digestion and absorption, and cholesterol metabolism (Figure 6B).
Differentially expressed proteins in plasma exosomes between lung squamous cell carcinoma and lung adenocarcinoma
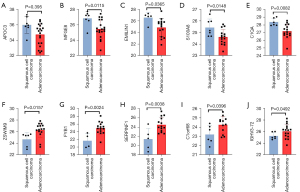
The expression levels of APOC3, MFGE8, EMILIN1, S100A8, and C1QA were significantly higher in patients with lung squamous cell carcinoma compared to patients with lung adenocarcinoma (P<0.05). Meanwhile, the expression of ZSWIM9, FYB1, SERPINF1, C1orf68, MASP2, and IGHV3-72 was significantly higher in patients with lung adenocarcinoma compared to patients with lung squamous cell carcinoma (P<0.05; Figure 7).
Among the 26 patients, all female patients presented with lung adenocarcinoma, and about half of all male patients presented with lung adenocarcinoma. For male patients, the expression of APOC3 and MFGE8 was significantly different between lung squamous cell carcinoma and lung adenocarcinoma patients.
Univariate and multivariate logistic regression in classifying samples
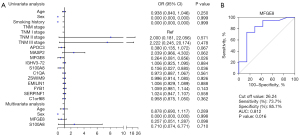
Univariate and multivariate logistic regression models were applied to identify the protein selectivity in classifying lung squamous cell carcinoma and adenocarcinoma (Figure 8). Univariate analyses demonstrated that MFGE8 and S100A8 were associated with the occurrence of lung squamous cell carcinoma. Multivariate analyses revealed that MFGE8 was associated with the occurrence of squamous cell carcinoma (P<0.1).
ROC curve
The ROC curve presents the sensitivity and specificity of MFGE8 levels in plasma-derived exosomes to screen lung squamous cell carcinoma and lung adenocarcinoma. The area under the curve (AUC) value was 0.812 and the cut-off value obtained from the ROC curve was 26.
Differentially expressed proteins in plasma exosomes from lung cancer patients with different pathological stage
There were 12 differentially expressed proteins in plasma exosomes derived from lung cancer patients with different pathological stages (Figure 9A-9L). Among them, IGHV5-51, IGHV3-74, and HBB were highly expressed in plasma exosomes from lung cancer patients in TNM stage II compared to patients in TNM stage I. Furthermore, the expression of CORO1A was higher in plasma exosomes from lung cancer patients in TNM stage III compared to patients in TNM stage I. In addition, F11, RBP4, and CORO1A expression was higher in plasma exosomes from TNM stage III patients compared to TNM stage II patients.
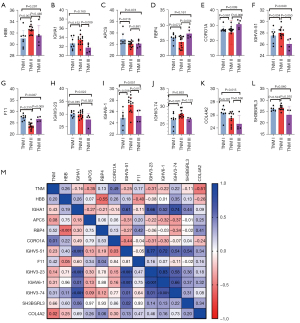
Conversely, RBP4 and APCS expression was relatively decreased in plasma exosomes from TNM stage II patients compared to stage I patients. Stage III patients had lower IGHV5-51, COL4A2, IGHV3-23, and SH3BGRL3 expression compared to stage I patients. IGHA1, IGHV6-1, IGHV5-51, IGHV3-23, and SH3BGRL3 showed lower expression in TNM stage III patients compared to stage II patients.
As shown in Figure 9M, CORO1A was positively correlated with the TNM stage of patients, while COL4A2 was negatively correlated with the TNM stage of patients.
Discussion
It is crucial to identify biomarkers which may be used to predict overall survival and recurrence (25). Tumor-derived exosomes communicate with their local environment through the proteins, miRNAs, and RNAs they carry and are critical for tumorigenesis, invasion, and migration (13-15). We evaluated the prognostic potential of secreted membrane binding proteins in a prospective cohort of nonselective plasma from NSCLC patients. The top 20 most abundantly expressed proteins in the plasma-derived exosomes were mainly associated with cholesterol metabolism in our study. Lipids have been reported to affect the immunity of patients with lung cancer (26). Furthermore, Ros-Mazurczyk et al. (27) suggested that the serum lipid profile might be discriminatory in patients with lung cancer and healthy controls. The multimarker models with the largest area under the curve in the cohort of patients with all lung cancer histological subtypes and in the cohort of patients with adenocarcinoma only covered 10 markers. In squamous cell cancer and SCLC, multimarker models did not exceed CD151 as an individual marker in separating patients with cancer from healthy controls (28). In addition, Niu et al. (29) used quantitative proteomics to investigate important pathways and functional categories in patients with metastatic and non-metastatic NSCLC, and healthy donors. Bioinformatics analysis showed that there was a good distinction between lipopolysaccharide binding proteins (LBPs) in the exosomes of patients with metastatic and non-metastatic NSCLC (29). The lipid synthesis pathway has been shown to be activated in cancer (30) and lipid metabolism in cancer can influence the lipid composition of exosomes derived from cancer cells (31).
Kuang et al. (24) also performed proteomics analyses on the plasma exosomes of patients with malignant and benign pulmonary nodules, and identified that the top 20 most abundant proteins in the plasma exosomes of people with malignant pulmonary nodules were AMBP, APOA1, F5, HABP2, FGA, F9, F10, APOE, FGG, LGALS3BP, ACTG1, FGB, C4, PA, IGHG1, ITIH3, ITIH1, C4A, F2, ALB, and ITIH2 (21). Among them, 7 proteins, including APOA1, FGA, APOE, FGG, FGB, C4BPA, and ALB, overlapped with the top 20 abundant proteins in the plasma exosomes of lung adenocarcinoma and lung squamous cell carcinoma patients found in this current study. Among the 7 proteins, APOA1 and APOE were associated with cholesterol metabolism. In a previous study, Borgquist et al. (32) suggested that the serum levels of apolipoproteins are associated with an increased risk of lung cancer. FGA, FGG, and FGB are associated with coagulation and it has been reported that cholesterol metabolism is important for the progression of cancer (33). Furthermore, it has been suggested that dysfunctional coagulation systems may exist in certain people with cancer (34).
The GO analysis and KEGG pathway analysis in the present study revealed that the target genes identified are significantly enriched in cholesterol metabolism, complement and coagulation cascade pathways. The tumor growth was related to the availability of the cholesterol (35). The reprogramming of the cholesterol metabolism and accumulation of cholesterol is a feature of NSCLC (36). Cell membrane cholesterol is critical for cancer cell proliferation and survival (37). Decreased cell membrane cholesterol inhibits the cell proliferation in NSCLC (38).
Dysregulated cholesterol homeostasis was related to the poor prognosis of the NSCLC (39), as abnormal serum cholesterol levels can cause immune dysfunction and reduced tumor cell proliferation (40,41). The expression of related components of high density lipoproteins (HDL) are upregulated in patients with adenocarcinoma and squamous cell carcinoma, and it is not clear whether these components are common in the plasma of healthy subjects. Previously, Pedersen et al. (42) found that several metabolites were altered in the pre-treatment serum samples of small-cell lung cancer patients compared to healthy individuals. Disordered lipid metabolism is featured by altered lipid metabolites (43). We speculate that the expression of HDL-related proteins in the plasma of NSCLCs patients may be higher than in healthy subjects.
As we noticed, the expression of APOC3, MFGE8, EMILIN1, S100A8, and C1QA was higher in patients with lung squamous cell carcinoma compared to patients with lung adenocarcinoma. Apolipoprotein C3 (APOC3) is an anti-aging protein strongly associated with age (44). It is located on the surface of lipoprotein particles and plays an important role in the regulation of triglycerides. A recent research has shown that APOC3 protein expression in plasma increases the risk of cardiovascular disease (45). Paiva et al. (46) displayed that APOC3 overexpression may lead to changes in liver inflammation, hepatocyte apoptosis, oxidative stress, and increased liver lipid content. Recently, a study demonstrated that APOC3 may be associated with the development of lung cancer. Wang et al. (47) suggested that APOC3 may be a prognostic biomarker for hepatocellular carcinoma (HCC). Milk fat globule-epidermal growth factor 8 (MFGE8) is a lipophilic glycoprotein that is widely involved in cell interactions and plays an important role in inflammatory injury, fibrosis, tumor development, and other diseases (48). Tian et al. (49) highlighted that MFGE8, as a COX-2-related gene, is involved in the regulation of tumor lung colonization in triple negative breast cancer (TNBC), and that silencing MFGE8 expression in TNBC cells significantly restored sensitivity to selective inhibitors of COX-2 both in vitro and in vivo. In lung cancer, Luo et al. (50) demonstrated that MFGE8, as a tumor antigen, may contribute to enhancing the T cell response. In this current study, the expression levels of MFGE8 were significantly higher in patients with lung squamous cell carcinoma compared to patients with lung adenocarcinoma and the difference at gene level might provide a deeper understanding in the mechanism. The EMILIN protein family the family of extracellular matrix glycoproteins that are widely distributed in connective tissues and play an important role in maintaining vascular, lymphatic, and skin homeostasis. A study showed that the EMILIN family can induce tumor cell apoptosis by mimicking classical extracellular apoptosis pathways, and may be involved in regulating tumor angiogenesis by interacting with other extracellular matrix components or cell surface molecules (51). A Gene expression study in human NSCLC revealed that increased expression of EMILIN1 was associated with low proliferation of tumor cells (52). S100A8 belongs to the S100 protein family which is closely related to the regulation of intracellular Ca2+, cell proliferation, differentiation, movement, and apoptosis, and other cell functions (53). In the process of studying the effect of S100A8 on lung cancer, Kinoshita et al. (54) found that S100A8 could induce the activation of myeloperoxidase (MPO), and a novel monoclonal antibody against S100A8 effectively prevented lung cancer metastasis. Furthermore, the sensitivity of drug-resistant NSCLC cells to gefitinib may be related to the regulation of S100A8 (55). Currently, there is a paucity of data regarding differences in plasma S100A8 expression between lung adenocarcinoma and lung squamous cell carcinoma. Complement C1q is an activator of the classical pathway. However, it is now recognized that C1q can perform functions unrelated to complement activation (56). Bulla et al. found that compared with wild-type (WT) or C3-or C5-deficient mice, C1q-deficient (C1qa−/−) mice with a syngeneic B16 melanoma had more prolonged survival and slower tumor growth rate, confirming the role of locally synthesized C1q could promote tumor growth (56).
Our results revealed that the expression of ZSWIM9, FYB1, SERPINF1, C1orf68, MASP2, and IGHV3-72 was higher in patients with lung adenocarcinoma compared to patients with lung squamous cell carcinoma. SWIM is a novel zinc finger-like domain with a Zn-chelated CxCxnCxH motif. At present, studies on ZSWIM and lung cancer have mainly focused on ZSWIM5. No relationship between ZSWIM9 and lung cancer has been reported. FYN binding protein 1 (FYB1), named adhesion and degranulation-promoting adapter protein (ADAP), is required for the activation of T cells (57). A recent study demonstrated that ADAP played a vital role in the activation of T cells and adhesion of β2 integrin in cells induced by infection or chemokines (58). In addition, ADAP can be expressed on primary natural killer cells and lymphocyte-activated killer cells stimulated by interleukin-2, leading to increased antitumor responses (59). ADAP may contribute to improve the prognosis of patients with NSCLC (60). SERPINF1 is expressed in quiescent cells and contains various frontal functions including anti-tumor, anti-angiogenesis, and neurotrophic characteristics (61). The migration and apoptotic resistance of endothelial cells are inhibited by SERPINF via the FAS/FASL or the p38 MAPK pathway. We previously demonstrated that SERPINF1 is differentially expressed in lung cancer cell lines and has predictive significance (62). In this study, we showed that SERPINF1 expression was higher in lung adenocarcinoma compared to lung squamous cell carcinoma, which confirmed the above results. The C1orf68 gene encodes the skin specific protein 32 (63) and current literature on C1orf68 are mainly focused on skin diseases (63). This study is the first to confirm the differential expression of C1orf68 in lung cancer. MASP2 is the central protease molecule of the complement system, and its expression is closely related to the occurrence and development of various tumors. MASP2 can be used as a biomarker for colorectal cancer, HCC, esophageal squamous cell carcinoma, lymphatic carcinoma, and other malignancies, and has great value in predicting the progression and prognosis of these diseases (64). Indeed, a study has shown that high serum MASP2 levels are associated with recurrent tumors and poor survival, demonstrating the independent prognostic value of MASP2 (65). Through genome and transcriptome analyses, Zengin et al. found that MASP2 was associated with lung adenocarcinoma (66), which was consistent with our results. Ighv3-72 belongs to the subgroup of IGHV3. Currently, research on the role of IGHV3 in disease has focused on hematological tumors such as lymphoma and leukemia. The current study is the first to confirm the differential expression of the IGHV3 family in lung cancer, providing a possible approach for clinical treatment. In this study, univariate and multivariate logistic regression was performed to identify the proteins that play an important role in the different types of NSCLC, different genders, and patients with genetic mutations. We found that MFGE8 and S100A8 were downregulated in adenocarcinomas. MFGE8 was originally identified as a breast cancer marker (67), but has since been associated with multiple tumors (68). MFGE8 plays major roles in tumor cell growth and proliferation (69) and is significantly upregulated in different types of squamous cell carcinomas, such as oral squamous cell carcinoma and esophageal squamous cell carcinoma (70). S100A8 is also a tumor suppressor in both squamous cell carcinoma and adenocarcinoma, however, until now there have been no reports related to the differential expression of MFGE8 and S100A8 in the plasma of squamous cell carcinoma and adenocarcinoma patients. The univariate logistic regression analyses revealed that TPI1, SDCBP, COL6A2, C1orf68, and ANXA2 were associated with the gender of the patients. However, to date, no other studies have shown a gender difference in the expression of these proteins in tumors. The L858R mutation and the exon 19 deletion are common EGFR mutations. It has been reported that the L858R mutation can promote cancer development through the CXCL12-CXCR4 pathway (71), but the molecular mechanisms in plasma exosomes remain unclear. Univariate analysis revealed that ITIH4, IGHM, and HPR expression were significantly different in patients with and without L858R mutation, however, no significant difference was detected in the multivariate analyses. To date, no studies have examined the effects of ITIH4, IGHM, and HPR expression on the L858R mutation in the NSCLC and future studies are warranted to verify these results.
The expression of MFGE8 has been associated with the prognosis of the patients with lung squamous cell carcinoma (70) and this is consistent with our results. However, the role of MFGE8 in lung squamous cell carcinoma remains to be elucidated.
This study demonstrated that the levels of CORO1A, IGHV5-51, IGHV3-23, COL4A2, and SH3BGRL3 were differentially expressed in patients with TNM stage I, II, and III cancer. CORO1A, which encodes TRP-ASP (WD) repeat proteins and is involved in a variety of cellular processes (72), has been shown to display predictive value for malignant outcomes (73). CORO1A deficiency can impair the homeostasis of peripheral cell (74). IGHV, also known as the unique type (idiotype), is a tumor specific antigen. Idiotypic types are the first proven immunotherapeutic targets that can induce tumor-specific immune responses, and their antitumor effects have been already demonstrated in animal and clinical trials. At present, studies on the IGHV family have mainly focused on blood system diseases, and a study has confirmed that IGHV 3 and IGHV4 can participate in the occurrence and development of blood diseases (75). In this study, multiple IGHV family proteins showed a different expression in lung cancer, suggesting that IGHV may play a certain role in the occurrence of lung cancer. Coagulation factor XI is also known as F11 and it can trigger the middle phase of the intrinsic pathway of blood coagulation by activating factor IX (76). Factor XI is initially synthesized in the liver, and form a complex with high molecular weight kininogen, then circulates as a disulfide bond-linked dimer in the plasma. It is converted into XIa on the platelet surface (77). IXa, which is converted by factor XI, subsequently activates factor X into Xa (78). Type 4-α-2 collagen (COL4A2) is essential for the function and stability of the vascular basement membrane (79). A report has confirmed that COL4A2 is involved in the development of tumors of the reproductive system, such as prostate cancer, epithelial ovarian cancer, uterine leiomyoma, and breast cancer (80). To date, there is a lack of literature supporting the role of COLA42 in lung cancer. The human SH3 domain combined with glutamate-rich like 3 (SH3BGRL3) gene is highly conserved in phylogeny and extensively expressed in human tissues. However, its function remains largely unknown. This protein has reported to be overexpressed in some tumors and a recent study suggested that it may be involved with members of the EGFR family (81). There is a positive feedback pathway between SH3BGRL3 and STAT3, which promotes tumor formation (82).
There were the limitations to this study. First, the association between high expression of plasma exosome proteins and prognosis of adenocarcinoma and squamous cell carcinoma cannot be determined owing to the small sample size which does not allow us infer causation certainly. Therefore, the sample size should be increased and extensive studies involving multiple centers should be conducted. Second, there were no normal healthy subjects analyzed and future investigations should recruit a set of healthy individuals to assess the differential expression of proteins in lung cancer patients. Third, the follow-up and the chemotherapy status of the patients were not considered in this report. It would be interesting to examine whether chemotherapy affects the levels of the differentially expressed proteins identified in our experience.
Conclusions
This study detailed the exosomal protein profiles at different stages of NSCLC and identified possible biomarkers candidate for use in clinical diagnosis. The results showed that the expression of APOC3, MFGE8, EMILIN1, S100A8, and C1QA was higher in patients with squamous cell carcinoma compared to those with adenocarcinoma. Conversely, the expression of ZSWIM9, FYB1, SERPINF1, C1orf68, MASP2, and IGHV3-72 was higher in patients with adenocarcinoma compared to those with squamous cell carcinoma. There were 11 differential expressed proteins in plasma exosomes from male and female lung cancer patients and 12 from different pathological lung cancer stage. Furthermore, 8 differentially expressed proteins in plasma exosomes were noticed in bronchogenic carcinoma with or without the L858R mutation.
Future direction
Recently, exosomes have been identified as a extracellular cargo. The exosomes have played important role in the microenvironment of the tumor through transferring miRNAs, RNAs, proteins. The content of the exosomes are associated with the functions of the exosomes. It was suggested that the bioactive molecules within tumor derived exosomes might be closely related to the prognosis and the immune status of the patients. Therefore, the analysis of exosomal protein content and the identification of potential biomarkers are important for exploring the prognosis of tumors or identifying targets. In the future, we will link the clinical information of patients with the protein content of exosomes to better identify targeted biomarkers.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-467/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-467/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-467/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee of the Shanghai Pulmonary Hospital (No. K20-197Y) approved the study protocol, and all patients provided written informed consent. The institutional review boards at Shanghai Pulmonary Hospital reviewed all protocols. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All consent documentation adhered to the Clinical Proteomic Tumor Analysis Consortium (CPTAC) guidelines.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med (Lond) 2018;18:s41-6. [Crossref] [PubMed]
- Wang L, Ma Q, Yao R, et al. Current status and development of anti-PD-1/PD-L1 immunotherapy for lung cancer. Int Immunopharmacol 2020;79:106088. [Crossref] [PubMed]
- Buddharaju LNR, Ganti AK. Immunotherapy in lung cancer: the chemotherapy conundrum. Chin Clin Oncol 2020;9:59. [Crossref] [PubMed]
- Jiang F, Zhou XY, Huang J. The value of surface enhanced laser desorption/ionization-time of flight mass spectrometry at the diagnosis of non-small cell lung cancer: a systematic review. Technol Cancer Res Treat 2014;13:109-17. [Crossref] [PubMed]
- Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin 2004;54:8-29. [Crossref] [PubMed]
- Geyer PE, Holdt LM, Teupser D, et al. Revisiting biomarker discovery by plasma proteomics. Mol Syst Biol 2017;13:942. [Crossref] [PubMed]
- Aslam B, Basit M, Nisar MA, et al. Proteomics: Technologies and Their Applications. J Chromatogr Sci 2017;55:182-96. [Crossref] [PubMed]
- Kok VC, Yu CC. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int J Nanomedicine 2020;15:8019-36. [Crossref] [PubMed]
- Fry WA, Phillips JL, Menck HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States. Cancer 1999;86:1867-76. [Crossref] [PubMed]
- Chen R, Xu X, Qian Z, et al. The biological functions and clinical applications of exosomes in lung cancer. Cell Mol Life Sci 2019;76:4613-33. [Crossref] [PubMed]
- Xu K, Zhang C, Du T, et al. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother 2021;134:111111. [Crossref] [PubMed]
- Zhou J, Wang H, Sun Q, et al. miR-224-5p-enriched exosomes promote tumorigenesis by directly targeting androgen receptor in non-small cell lung cancer. Mol Ther Nucleic Acids 2021;23:1217-28. [Crossref] [PubMed]
- Huang WT, Chong IW, Chen HL, et al. Pigment epithelium-derived factor inhibits lung cancer migration and invasion by upregulating exosomal thrombospondin 1. Cancer Lett 2019;442:287-98. [Crossref] [PubMed]
- Chen X, Wang Z, Tong F, et al. lncRNA UCA1 Promotes Gefitinib Resistance as a ceRNA to Target FOSL2 by Sponging miR-143 in Non-small Cell Lung Cancer. Mol Ther Nucleic Acids 2020;19:643-53. [Crossref] [PubMed]
- Maia J, Caja S, Strano Moraes MC, et al. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front Cell Dev Biol 2018;6:18. [Crossref] [PubMed]
- Gu H, Ji R, Zhang X, et al. Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol Med Rep 2016;14:3452-8. [Crossref] [PubMed]
- Wang H, Wang L, Pan H, et al. Exosomes Derived From Macrophages Enhance Aerobic Glycolysis and Chemoresistance in Lung Cancer by Stabilizing c-Myc via the Inhibition of NEDD4L. Front Cell Dev Biol 2021;8:620603. [Crossref] [PubMed]
- Stahl PD, Raposo G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology (Bethesda) 2019;34:169-77. [Crossref] [PubMed]
- Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013;2:
10.3402/jev.v2i0.20360 . doi:10.3402/jev.v2i0.20360 .10.3402/jev.v2i0.20360 - Tai YL, Chen KC, Hsieh JT, et al. Exosomes in cancer development and clinical applications. Cancer Sci 2018;109:2364-74. [Crossref] [PubMed]
- Cheng L, Sharples RA, Scicluna BJ, et al. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles 2014;3:
10.3402/jev.v3.23743 . doi:10.3402/jev.v3.23743 .10.3402/jev.v3.23743 - Sandfeld-Paulsen B, Jakobsen KR, Bæk R, et al. Exosomal Proteins as Diagnostic Biomarkers in Lung Cancer. J Thorac Oncol 2016;11:1701-10. [Crossref] [PubMed]
- Kuang M, Tao X, Peng Y, et al. Proteomic analysis of plasma exosomes to differentiate malignant from benign pulmonary nodules. Clin Proteomics 2019;16:5. [Crossref] [PubMed]
- Li X, Wang L, Wang L, et al. Single-Cell Sequencing of Hepatocellular Carcinoma Reveals Cell Interactions and Cell Heterogeneity in the Microenvironment. Int J Gen Med 2021;14:10141-53. [Crossref] [PubMed]
- Karayama M, Inui N, Inoue Y, et al. Increased serum cholesterol and long-chain fatty acid levels are associated with the efficacy of nivolumab in patients with non-small cell lung cancer. Cancer Immunol Immunother 2022;71:203-17. [Crossref] [PubMed]
- Ros-Mazurczyk M, Jelonek K, Marczyk M, et al. Serum lipid profile discriminates patients with early lung cancer from healthy controls. Lung Cancer 2017;112:69-74. [Crossref] [PubMed]
- Wang N, Song X, Liu L, et al. Circulating exosomes contain protein biomarkers of metastatic non-small-cell lung cancer. Cancer Sci 2018;109:1701-9. [Crossref] [PubMed]
- Niu L, Song X, Wang N, et al. Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci 2019;110:433-42. [Crossref] [PubMed]
- Bian X, Liu R, Meng Y, et al. Lipid metabolism and cancer. J Exp Med 2021;218:e20201606. [Crossref] [PubMed]
- Wang W, Bai L, Li W, et al. The Lipid Metabolic Landscape of Cancers and New Therapeutic Perspectives. Front Oncol 2020;10:605154. [Crossref] [PubMed]
- Borgquist S, Butt T, Almgren P, et al. Apolipoproteins, lipids and risk of cancer. Int J Cancer 2016;138:2648-56. [Crossref] [PubMed]
- Ding X, Zhang W, Li S, et al. The role of cholesterol metabolism in cancer. Am J Cancer Res 2019;9:219-27. [PubMed]
- Fei X, Wang H, Yuan W, et al. Tissue Factor Pathway Inhibitor-1 Is a Valuable Marker for the Prediction of Deep Venous Thrombosis and Tumor Metastasis in Patients with Lung Cancer. Biomed Res Int 2017;2017:8983763. [Crossref] [PubMed]
- Hoppstädter J, Dembek A, Höring M, et al. Dysregulation of cholesterol homeostasis in human lung cancer tissue and tumour-associated macrophages. eBioMedicine 2021;72:103578. [Crossref] [PubMed]
- Pan Z, Wang K, Wang X, et al. Cholesterol promotes EGFR-TKIs resistance in NSCLC by inducing EGFR/Src/Erk/SP1 signaling-mediated ERRα re-expression. Molecular Cancer 2022;21:1-17. [Crossref]
- Mollinedo F, Gajate C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: implications in tumor progression and therapy: Thematic Review Series: Biology of Lipid Rafts. J Lipid Res 2020;61:611-35. [Crossref] [PubMed]
- Hsu J-L, Leu W-J, Zhong N-S, et al. Autophagic Activation and Decrease of Plasma Membrane Cholesterol Contribute to Anticancer Activities in Non-Small Cell Lung Cancer. Molecules 2021;26:5967. [Crossref] [PubMed]
- Li JR, Zhang Y, Zheng JL. Decreased pretreatment serum cholesterol level is related with poor prognosis in resectable non-small cell lung cancer. Int J Clin Exp Pathol 2015;8:11877-83. [PubMed]
- Ma C, Wang X, Guo J, et al. Prognostic significance of preoperative serum triglycerides and high-density lipoproteins cholesterol in patients with non-small cell lung cancer: a retrospective study. Lipids Health Dis 2021;20:69. [Crossref] [PubMed]
- Kuzu OF, Noory MA, Robertson GP. The Role of Cholesterol in Cancer. Cancer Res 2016;76:2063-70. [Crossref] [PubMed]
- Pedersen S, Hansen JB, Maltesen RG, et al. Identifying metabolic alterations in newly diagnosed small cell lung cancer patients. Metabol Open 2021;12:100127. [Crossref] [PubMed]
- Long J, Zhang CJ, Zhu N, et al. Lipid metabolism and carcinogenesis, cancer development. Am J Cancer Res 2018;8:778-91. [PubMed]
- Reeskamp LF, Tromp TR, Stroes ESG. The next generation of triglyceride-lowering drugs: will reducing apolipoprotein C-III or angiopoietin like protein 3 reduce cardiovascular disease? Curr Opin Lipidol 2020;31:140-6. [Crossref] [PubMed]
- Kanter JE, Shao B, Kramer F, et al. Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes. J Clin Invest 2019;129:4165-79. [Crossref] [PubMed]
- Paiva AA, Raposo HF, Wanschel AC, et al. Apolipoprotein CIII Overexpression-Induced Hypertriglyceridemia Increases Nonalcoholic Fatty Liver Disease in Association with Inflammation and Cell Death. Oxid Med Cell Longev 2017;2017:1838679. [Crossref] [PubMed]
- Wang H, Fu Y, Da BB, et al. Single-Cell Sequencing Identifies the Heterogeneity of CD8+ T Cells and Novel Biomarker Genes in Hepatocellular Carcinoma. J Healthc Eng 2022;2022:8256314. [Crossref] [PubMed]
- Sinningen K, Thiele S, Hofbauer LC, et al. Role of milk fat globule-epidermal growth factor 8 in osteoimmunology. Bonekey Rep 2016;5:820. [Crossref] [PubMed]
- Tian J, Wang V, Wang N, et al. Identification of MFGE8 and KLK5/7 as mediators of breast tumorigenesis and resistance to COX-2 inhibition. Breast Cancer Res 2021;23:23. [Crossref] [PubMed]
- Luo L, Lv M, Zhuang X, et al. Irradiation increases the immunogenicity of lung cancer cells and irradiation-based tumor cell vaccine elicits tumor-specific T cell responses in vivo. Onco Targets Ther 2019;12:3805-15. [Crossref] [PubMed]
- Mongiat M, Buraschi S, Andreuzzi E, et al. Extracellular matrix: the gatekeeper of tumor angiogenesis. Biochem Soc Trans 2019;47:1543-55. [Crossref] [PubMed]
- Amor López A, Mazariegos MS, Capuano A, et al. Inactivation of EMILIN-1 by Proteolysis and Secretion in Small Extracellular Vesicles Favors Melanoma Progression and Metastasis. Int J Mol Sci 2021;22:7406. [Crossref] [PubMed]
- Wang S, Song R, Wang Z, et al. S100A8/A9 in Inflammation. Front Immunol 2018;9:1298. [Crossref] [PubMed]
- Kinoshita R, Sato H, Yamauchi A, et al. Newly developed anti-S100A8/A9 monoclonal antibody efficiently prevents lung tropic cancer metastasis. Int J Cancer 2019;145:569-75. [Crossref] [PubMed]
- Li G, Ma Y, Yu M, et al. Identification of Hub Genes and Small Molecule Drugs Associated with Acquired Resistance to Gefitinib in Non-Small Cell Lung Cancer. J Cancer 2021;12:5286-95. [Crossref] [PubMed]
- Bulla R, Tripodo C, Rami D, et al. C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation. Nat Commun 2016;7:10346. [Crossref] [PubMed]
- Parzmair GP, Gereke M, Haberkorn O, et al. ADAP plays a pivotal role in CD4+ T cell activation but is only marginally involved in CD8+ T cell activation, differentiation, and immunity to pathogens. J Leukoc Biol 2017;101:407-19. [Crossref] [PubMed]
- Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology 2012;135:268-75. [Crossref] [PubMed]
- Fostel LV, Dluzniewska J, Shimizu Y, et al. ADAP is dispensable for NK cell development and function. Int Immunol 2006;18:1305-14. [Crossref] [PubMed]
- Zhang X, Shi X, Zhao H, et al. Identification and Validation of a Tumor Microenvironment-Related Gene Signature for Prognostic Prediction in Advanced-Stage Non-Small-Cell Lung Cancer. Biomed Res Int 2021;2021:8864436. [Crossref] [PubMed]
- Liu JT, Chen YL, Chen WC, et al. Role of pigment epithelium-derived factor in stem/progenitor cell-associated neovascularization. J Biomed Biotechnol 2012;2012:871272. [Crossref] [PubMed]
- Lam DC, Girard L, Suen WS, et al. Establishment and expression profiling of new lung cancer cell lines from Chinese smokers and lifetime never-smokers. J Thorac Oncol 2006;1:932-42. [Crossref] [PubMed]
- Edqvist PH, Fagerberg L, Hallström BM, et al. Expression of human skin-specific genes defined by transcriptomics and antibody-based profiling. J Histochem Cytochem 2015;63:129-41. [Crossref] [PubMed]
- Maestri CA, Nisihara R, Mendes HW, et al. MASP-1 and MASP-2 Serum Levels Are Associated With Worse Prognostic in Cervical Cancer Progression. Front Immunol 2018;9:2742. [Crossref] [PubMed]
- Ytting H, Christensen IJ, Thiel S, et al. Serum mannan-binding lectin-associated serine protease 2 levels in colorectal cancer: relation to recurrence and mortality. Clin Cancer Res 2005;11:1441-6. [Crossref] [PubMed]
- Zengin T, Önal-Süzek T. Analysis of genomic and transcriptomic variations as prognostic signature for lung adenocarcinoma. BMC Bioinformatics 2020;21:368. [Crossref] [PubMed]
- Carrascosa C, Obula RG, Missiaglia E, et al. MFG-E8/lactadherin regulates cyclins D1/D3 expression and enhances the tumorigenic potential of mammary epithelial cells. Oncogene 2012;31:1521-32. [Crossref] [PubMed]
- Ko DS, Kim SH, Park JY, et al. Milk Fat Globule-EGF Factor 8 Contributes to Progression of Hepatocellular Carcinoma. Cancers (Basel) 2020;12:403. [Crossref] [PubMed]
- Zhao Q, Xu L, Sun X, et al. MFG-E8 overexpression promotes colorectal cancer progression via AKT/MMPs signalling. Tumour Biol 2017;39:1010428317707881. [Crossref] [PubMed]
- Yamazaki M, Maruyama S, Abé T, et al. MFG-E8 expression for progression of oral squamous cell carcinoma and for self-clearance of apoptotic cells. Lab Invest 2014;94:1260-72. [Crossref] [PubMed]
- Lüke F, Blazquez R, Yamaci RF, et al. Isolated metastasis of an EGFR-L858R-mutated NSCLC of the meninges: the potential impact of CXCL12/CXCR4 axis in EGFRmut NSCLC in diagnosis, follow-up and treatment. Oncotarget 2018;9:18844-57. [Crossref] [PubMed]
- Liu C, Wang Y, Zhang H, et al. Porcine coronin 1A contributes to nuclear factor-kappa B (NF-κB) inactivation during Haemophilus parasuis infection. PLoS One 2014;9:e103904. [Crossref] [PubMed]
- Zhao Y, Sun H, Zheng J, et al. Identification of predictors based on drug targets highlights accurate treatment of goserelin in breast and prostate cancer. Cell Biosci 2021;11:5. [Crossref] [PubMed]
- Yee CS, Massaad MJ, Bainter W, et al. Recurrent viral infections associated with a homozygous CORO1A mutation that disrupts oligomerization and cytoskeletal association. J Allergy Clin Immunol 2016;137:879-88.e2. [Crossref] [PubMed]
- Stamatopoulos K, Belessi C, Moreno C, et al. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood 2007;109:259-70. [Crossref] [PubMed]
- Gailani D, Renné T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol 2007;27:2507-13. [Crossref] [PubMed]
- Baglia FA, Walsh PN. Prothrombin is a cofactor for the binding of factor XI to the platelet surface and for platelet-mediated factor XI activation by thrombin. Biochemistry 1998;37:2271-81. [Crossref] [PubMed]
- Hopfner KP, Brandstetter H, Karcher A, et al. Converting blood coagulation factor IXa into factor Xa: dramatic increase in amidolytic activity identifies important active site determinants. EMBO J 1997;16:6626-35. [Crossref] [PubMed]
- Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet 2012;21:R97-110. [Crossref] [PubMed]
- Egorova A, Pyankov I, Maretina M, et al. Peptide Nanoparticle-Mediated Combinatorial Delivery of Cancer-Related siRNAs for Synergistic Anti-Proliferative Activity in Triple Negative Breast Cancer Cells. Pharmaceuticals (Basel) 2021;14:957. [Crossref] [PubMed]
- Chiang CY, Pan CC, Chang HY, et al. SH3BGRL3 Protein as a Potential Prognostic Biomarker for Urothelial Carcinoma: A Novel Binding Partner of Epidermal Growth Factor Receptor. Clin Cancer Res 2015;21:5601-11. [Crossref] [PubMed]
- Nie Z, Cheng D, Pan C, et al. SH3BGRL3, transcribed by STAT3, facilitates glioblastoma tumorigenesis by activating STAT3 signaling. Biochem Biophys Res Commun 2021;556:114-20. [Crossref] [PubMed]



