
Comparison of perioperative outcomes among non-small cell lung cancer patients with neoadjuvant immune checkpoint inhibitor plus chemotherapy, EGFR-TKI, and chemotherapy alone: a real-world evidence study
Introduction
Surgical resection remains the most effective treatment strategy for potentially resectable non-small cell lung cancer (NSCLC). The 5-year overall survival rates among surgically resected NSCLC range from 83% for stage IA disease to 26% for stage IIIB disease, with most mortality patients undergoing postoperative relapse (1). Utilizing neoadjuvant chemotherapy is one approach that attempts to augment the outcomes afforded by surgery alone. A limitation of this strategy, however, is that the actual proportion of patients achieving a pathological complete response (pCR) is low, and there is a considerable incidence of toxicity (2,3).
Immune checkpoint inhibitors (ICIs) that block the programmed death-1 (PD-1) pathway have revolutionized the cancer treatment landscape (4-6). The monotherapy of nivolumab accomplished a pCR in 15% resected tumor. Interestingly, the combination of ICI with chemotherapy has shown a significant survival advantage in metastatic NSCLC, generating considerable enthusiasm for the use of ICI plus chemotherapy when treating early-stage resectable NSCLC (7). Incorporating ICI and chemotherapy into the management of early-stage NSCLC is attractive because the primary tumor may release neoantigens from dying tumor cells and activate tumor-specific T cells before surgical resection (8,9). Previous clinical trials have found that neoadjuvant ICI plus chemotherapy were associated with a higher proportion of patients achieving pCR (10,11). Recently, the CheckMate 816 study, the first of several phase III trials evaluating neoadjuvant ICI plus chemotherapy, resulted in a significantly longer median event-free survival (31.6 vs. 20.8 months) and higher percentage of pCR (24.0% vs. 2.2%) than chemotherapy alone in resectable NSCLC (12). Furthermore, the treatment-related adverse events of neoadjuvant ICI were observed to be acceptable and not associated with surgery delays.
In contrast to traditional platinum-based neoadjuvant chemotherapy, ICI that regulate the immune-inhibitory pathway of patients to release their own anti-tumor immunity has attracted special attention on the perioperative outcomes. The unique mechanism of neoadjuvant ICI plus chemotherapy may increase the risk of pneumonitis and adhesion, which can, theoretically, lead to technical challenges for lung resections (13). However, reports regarding the surgical technique and the perioperative outcomes of surgical resection after neoadjuvant ICI plus chemotherapy in NSCLC, especially in comparison with traditional neoadjuvant chemotherapy, are scarce. Moreover, epidermal growth factor receptor (EGFR) mutation is present in 39% to 59% NSCLC patients in East Asia population, which are predictive of response to EGFR-tyrosine kinase inhibitors (TKI) (14-17). Zhong et al. reported that patients with neoadjuvant EGFR-TKI have significantly longer progression-free survival versus neoadjuvant chemotherapy (18).
Therefore, the objective of this study was to evaluate the perioperative complications and mortalities of surgical resection after neoadjuvant ICI plus chemotherapy and comprehensively compare these outcomes with neoadjuvant EGFR-TKI and neoadjuvant chemotherapy alone in patients with resectable NSCLC. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-476/rc).
Methods
Ethical statement
This is a retrospective, sing-center study. We retrospectively enrolled patients who underwent neoadjuvant ICI plus chemotherapy, neoadjuvant EGFR-TKI, and neoadjuvant chemotherapy alone from Shanghai Pulmonary Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Shanghai Pulmonary Hospital (No. L21-224), and written informed consent was waived due to the retrospective nature of the study.
Study population and procedures
A total of 42 patients with NSCLC who received neoadjuvant ICI plus chemotherapy between January 2018 and December 2020 were reviewed. Patients with history of previous cancer, positive tumor driver mutation, and ICI-only neoadjuvant therapy were excluded. All enrolled patients were diagnosed with clinical stage IB to IIIB disease according to the 8th edition of the tumor, node, metastasis (TNM) classification for lung cancer. To compare the perioperative outcomes of neoadjuvant ICI plus chemotherapy with neoadjuvant EGFR-TKI and neoadjuvant chemotherapy, 54 patients who received neoadjuvant EGFR-TKI and 98 patients who received neoadjuvant chemotherapy during the same period also were evaluated.
All patients underwent a standard staging workup before treatment, including a chest computed tomography (CT) scan, abdominal CT scan, and magnetic resonance imaging of the brain. Positron emission tomography (PET)/CT scan and endobronchial ultrasound (EBUS)-guided transbronchial needle aspirations were also performed when necessary. Patients with central tumor and hilar or mediastinal lymphadenopathy on CT scan were required to undergo a PET-CT scan or EBUS examination for precise staging before neoadjuvant treatment. In addition, a tumor biopsy was mandatory for histological diagnosis and mutation detection before neoadjuvant treatment. The strategy of neoadjuvant treatment was decided upon patients’ profile, driver mutation status, and discussion by a group of oncologists, surgeons, and radiologists together. Patients in the chemotherapy alone group received 2–4 of cycles of platinum-based doublet chemotherapy before surgery. Additionally, the same regimens of adjuvant chemotherapy for two cycles were performed after complete resection or until disease progression or unacceptable toxicity. For patients harboring EGFR mutations (EGFR-TKI group), standard doses of EGFR-TKIs, such as gefitinib, icotinib, erlotinib, and afatinib, were administered daily during 3 to 6 weeks before surgery. Postoperative adjuvant treatment using either EGFR-TKI or platinum-based chemotherapy was chosen on the basis of the patient’s condition and the clinical experience of the medical oncologist.
Eligible patients in the neoadjuvant ICI group received pembrolizumab (200 mg) and platinum-based doublet chemotherapy on day 1 of each 21-day cycle, with a total of 2–4 cycles before surgical resection. Generally, surgery was performed approximately 4 weeks after the 1st day of the last treatment cycle. When patients were unable to tolerate the treatment-related adverse effects, or there was local disease progression according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), the patients were taken immediately to surgery. A repeat chest CT scan and pulmonary function test were required within 7 days before surgery. Resection of the primary tumor and systematic mediastinal lymph node (LN) dissection were performed. Postoperative ICI plus chemotherapy or chemotherapy alone was administered within 3 to 8 weeks after complete resection were administered. The pathological response was assessed using routine hematoxylin and eosin staining by measuring the percentage of residual viable tumor in the resected primary tumor. Major pathologic response (MPR) was defined as no more than 10% viable tumor cells in the resected primary tumor (19).
Surgical techniques
Video-assisted thoracoscopic surgery (VATS) was the preferred surgical approach at our center. Detailed surgical procedures for VATS lobectomy and pneumonectomy have been described in our previous studies (20,21). Sleeve lobectomy generally was performed at our center for centrally located tumors to avoid pneumonectomy (22,23). For uniportal VATS sleeve lobectomy, a 3–5 cm incision at the 4th intercostal space of the anterior axillary line was drawn. An intraoperative frozen section was required to confirm that the tumor was free of proximal and distal margins. A systematic hilar and mediastinal LN dissection was performed before the anastomosis. The inferior pulmonary ligament was always released to reduce anastomotic tension. Subsequently, end-to-end bronchial anastomosis was performed using continuous sutures with a 3-0 Prolene running suture (Video 1).
Variables studied and statistical analysis
The demographic information, clinical and pathologic characteristics, data about neoadjuvant therapy, surgical outcomes, and perioperative complications were collected from the medical record system. All clinical and pathological classifications were recorded in accordance with the 8th edition of the TNM staging system (1). A peripheral tumor was defined as a tumor located in the outer 3rd of the lung on the CT scan. The Charlson Comorbidity Index (CCI) was used to describe comorbidities (24). Perioperative mortality was defined as death from any cause within 30 days after surgery. Prolonged air leakage was defined as lasting for more than 7 days after surgery. Postoperative complications were evaluated using the Common Terminology Criteria for Adverse Events (version 5.0).
Continuous variables are presented as the mean ± standard deviation or the median value with the interquartile range (IQR). Categorical variables are presented as frequency and percentage. Baseline characteristics and perioperative outcomes were compared among the ICI plus chemotherapy group, EGFR-TKI group, and chemotherapy alone group using 1-way analysis of variance for continuous variables and Pearson chi-square test or Fisher exact test for categorical variables. A 2-sided P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 26.0 (IBM Corporation, Armonk, NY, USA).
Results
Baseline characteristics
Between January 2018 and December 2020, 42 patients received neoadjuvant ICI plus chemotherapy followed by surgical resection. During the same period, 54 patients received neoadjuvant EGFR-TKI and 98 patients received neoadjuvant chemotherapy alone followed by surgical resection. The baseline characteristics of these three groups are listed and compared in Table 1.
Table 1
| Variables | ICI plus chemotherapy (N=42) | EGFR-TKI (N=54) | Chemotherapy alone (N=98) | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 61.3±9.1 | 59.3±9.2 | 60.7±7.3 | 0.470 |
| Gender, n (%) | <0.001 | |||
| Male | 40 (95.2) | 24 (44.4) | 83 (84.7) | |
| Female | 2 (4.8) | 30 (55.6) | 15 (15.3) | |
| Smoking history, n (%) | <0.001 | |||
| Never | 18 (42.9) | 44 (81.5) | 52 (53.1) | |
| Current/former | 24 (57.1) | 10 (18.5) | 46 (46.9) | |
| BMI (kg/m2), mean ± SD | 25.2±3.0 | 24.6±3.5 | 23.6±3.0 | 0.043 |
| FEV1% (of predicted), mean ± SD | 89.1±23.6 | 91.1±15.2 | 88.8±17.2 | 0.742 |
| Preoperative staging, n (%) | ||||
| PET-CT | 34 (81.0) | 29 (53.7) | 42 (42.9) | <0.001 |
| EBUS | 24 (57.1) | 21 (38.9) | 52 (53.1) | 0.143 |
| Tumor location, n (%) | 0.241 | |||
| Upper lobe | 26 (61.9) | 33 (61.1) | 60 (61.2) | |
| Middle lobe | 2 (4.8) | 7 (13.0) | 4 (4.1) | |
| Lower lobe | 14 (33.3) | 14 (25.9) | 34 (34.7) | |
| Central/peripheral lesion, n (%) | <0.001 | |||
| Central | 23 (54.8) | 9 (16.7) | 44 (44.9) | |
| Peripheral | 19 (45.2) | 45 (83.3) | 54 (55.1) | |
| Radiologic tumor size (cm), mean ± SD | ||||
| Before neoadjuvant treatment | 4.7±2.1 | 3.9±1.5 | 4.2±1.8 | 0.122 |
| After neoadjuvant treatment | 2.6±1.3 | 2.6±1.0 | 3.5±1.8 | 0.005 |
| Clinical TNM stage, n (%) | 0.003 | |||
| I | 1 (2.4) | 7 (13.0) | 5 (5.1) | |
| II | 1 (2.4) | 2 (3.7) | 18 (18.4) | |
| III | 40 (95.2) | 45 (83.3) | 75 (76.5) | |
| Radiologic response (RESCIST 1.1 criteria), n (%) | 0.001 | |||
| Partial response | 25 (59.5) | 23 (42.6) | 22 (22.4) | |
| Stable disease | 9 (21.4) | 21 (38.9) | 48 (49.0) | |
| Progressive disease | 0 (0) | 3 (5.6) | 8 (8.2) | |
| Unavailable | 8 (19.0) | 7 (13.0) | 20 (20.4) | |
| Histology, n (%) | <0.001 | |||
| Adenocarcinoma | 8 (19.0) | 53 (98.1) | 37 (37.8) | |
| Squamous cell carcinoma | 29 (69.0) | 0 (0) | 53 (54.1) | |
| Other/unspecific | 5 (11.9) | 1 (1.9) | 8 (8.2) | |
| Charlson Comorbidity Index, n (%) | 0.568 | |||
| 0 | 5 (11.9) | 9 (16.7) | 9 (9.2) | |
| 1 | 9 (21.4) | 13 (24.1) | 14 (14.3) | |
| 2 | 19 (45.2) | 22 (40.7) | 46 (46.9) | |
| 3 | 5 (11.9) | 8 (14.8) | 18 (18.4) | |
| 4 | 4 (9.5) | 2 (3.7) | 11 (11.2) | |
ICI, immune checkpoint inhibitor; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitors; SD, standard deviation; BMI, body mass index; FEV1, forced expiratory volume in one second; PET-CT, positron emission tomography-computed tomography; EBUS, endobronchial ultrasound; TNM, tumor, node, metastasis.
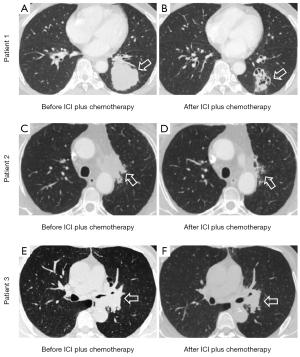
Patients in the ICI plus chemotherapy group and chemotherapy alone group had higher proportions of male patients (95.2% vs. 84.7% vs. 44.4%, P<0.001), higher proportions of smoking history (57.1% vs. 46.9% vs. 18.5%, P<0.001), more centrally located tumors (54.8% vs. 44.9% vs. 16.7%, P<0.001), and more advanced clinical TNM stages (stage II to III, 97.6% vs. 94.9% vs. 87.0%, P=0.003) compared with patients in the EGFR-TKI group. In addition, patients in the ICI plus chemotherapy and chemotherapy alone groups had and compared with patients in the EGFR-TKI group. Patients in the ICI plus chemotherapy group and EGFR-TKI group had similar radiologic tumor size with chemotherapy alone group before neoadjuvant treatment (4.7 vs. 3.9 vs. 4.2 cm, P=0.122), but significantly smaller tumor size after neoadjuvant treatment (3.5 vs. 2.6 vs. 2.6 cm, P=0.005). According to RESCIST version 1.1 criteria, 25 (59.5%), 23 (42.6%), and 22 (22.4%) patients achieved radiological response (P=0.001) in the ICI, EGFR-TKI, and chemotherapy groups, respectively. Representative radiologic images of the lesion before and after the neoadjuvant ICI plus chemotherapy treatment are shown in Figure 1. There were no significant differences among the three groups in terms of age, forced expiratory volume in 1 second of predicted value, body mass index, and CCI.
Perioperative outcomes
All patients underwent R0 resection and no intraoperative deaths were observed. Table 2 shows the perioperative outcomes of the three groups. The median interval times from last treatment to surgery were 36 (IQR, 31–41), 12 (IQR, 5–21), and 37 (IQR, 30–49) days for the ICI, EGFR-TKI, and chemotherapy groups (P<0.001), respectively. No treatment-related surgical delay was observed. Patients in the ICI plus chemotherapy group and chemotherapy alone group were associated with higher proportions of sleeve lobectomies compared with those in the EGFR-TKI group (33.3% vs. 21.4% vs. 11.1%, P=0.001). There were 37 cases (88.1%), 49 cases (90.7%), and 57 cases (58.2%) resected via VATS approach in the ICI, EGFR-TKI, and chemotherapy groups, respectively (P<0.001).
Table 2
| Perioperative outcomes | ICI plus chemotherapy (N=42) | EGFR-TKI (N=54) | Chemotherapy alone (N=98) | P value |
|---|---|---|---|---|
| Time from last treatment to surgery (days), median [IQR] | 36 [31–41] | 12 [5–21] | 37 [30–49] | <0.001 |
| Extent of resection, n (%) | 0.001 | |||
| Lobectomy | 19 (45.2) | 45 (83.3) | 49 (50.0) | |
| Bilobectomy | 6 (14.3) | 1 (1.9) | 19 (19.4) | |
| Sleeve lobectomy | 14 (33.3) | 6 (11.1) | 21 (21.4) | |
| Pneumonectomy | 3 (7.1) | 2 (3.7) | 9 (9.2) | |
| Surgical approach, n (%) | <0.001 | |||
| VATS | 37 (88.1) | 49 (90.7) | 57 (58.2) | |
| Thoracotomy | 4 (9.5) | 4 (7.4) | 36 (36.7) | |
| VATS convert to thoracotomy | 1 (2.4) | 1 (1.9) | 5 (5.1) | |
| Total number of examined LNs, mean ± SD | 15.3±8.4 | 14.1±7.4 | 16.2±7.9 | 0.271 |
| Examined N1 nodes | 7.7±4.5 | 6.4±4.2 | 7.3±4.0 | 0.249 |
| Examined N2 nodes | 9.6±5.3 | 8.3±4.2 | 9.4±5.3 | 0.335 |
| Surgical time (min), median [IQR] | 150 [120–180] | 120 [99–167] | 146 [120–180] | 0.041 |
| Estimated blood loss (mL), median [IQR] | 50 [50–100] | 50 [50–100] | 100 [50–150] | 0.846 |
| Required for postoperative transfusion, n (%) | 5 (11.9) | 8 (14.8) | 6 (6.1) | 0.197 |
| Postoperative stay (days), median [IQR] | 5 [4–7] | 3 [3–6] | 5 [4–7] | 0.164 |
| Perioperative mortality, n (%) | 1 (2.4) | 0 (0) | 3 (3.1) | 0.440 |
| Postoperative complications, n (%) | ||||
| Any complications | 7 (16.7) | 3 (5.6) | 24 (24.5) | 0.013 |
| Bronchopleural fistula | 2 (4.8) | 0 (0) | 1 (1.0) | 0.144 |
| Hemothorax | 0 (0) | 0 (0) | 2 (2.0) | 0.372 |
| Chylothorax | 0 (0) | 0 (0) | 2 (2.0) | 0.372 |
| Prolonged air leak | 2 (4.8) | 1 (1.9) | 3 (3.1) | 0.714 |
| Pneumonia | 3 (7.1) | 1 (1.9) | 16 (16.3) | 0.014 |
| Pulmonary embolism | 0 (0) | 1 (1.9) | 0 (0) | 0.272 |
ICI, immune checkpoint inhibitor; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitors; IQR, interquartile range; VATS, video-assisted thoracoscopic surgery; LN, lymph node; SD, standard deviation.
Patients in the ICI plus chemotherapy group and chemotherapy alone group had longer operative time than those in the EGFR-TKI group (150 vs. 146 vs. 120 minutes, P=0.041). The number of examined LNs, estimated blood loss, rates of postoperative transfusion, and postoperative hospital stay were evenly distributed among the three groups. The perioperative mortality within 30 days was 2.4%, 0%, and 3.1% for the ICI, EGFR-TKI, and chemotherapy groups, respectively (P=0.440). One patient in the ICI plus chemotherapy group died due to bronchopleural fistula after sleeve lobectomy. Three patients in the chemotherapy alone group died due to chylothorax, food aspiration, and hemothorax, respectively.
The overall postoperative complications were significantly higher in the ICI plus chemotherapy group and chemotherapy alone group than the EGFR-TKI group (16.7% vs. 24.5% vs. 5.6%, P=0.013). Patients in the ICI plus chemotherapy group and EGFR-TKI group were associated with a significantly lower rate of pneumonia than patients in the chemotherapy alone group (7.1% vs. 1.9% vs. 16.3%, P=0.014). Hemothorax, chylothorax, prolonged air leak, and pulmonary embolism were similar among the three groups. Bronchopleural fistula occurred more frequently in the ICI plus chemotherapy group than the EGFR-TKI and chemotherapy alone groups (4.8% vs. 0% vs. 1.0%), but the difference was not statistically significant (P=0.144).
Tumor response to neoadjuvant treatment
A higher proportion of patients in the ICI plus chemotherapy group achieved pCR (17/42, 40.5%) and MPR (30/42, 71.4%) compared with patients in the EGFR-TKI group (pCR: 6/54, 11.1%; MPR: 11/54, 20.4%) and chemotherapy alone group (pCR: 6/98, 6.1%; MPR: 14/98, 14.3%) (Table 3). Furthermore, the proportion of downstaging of nodal status from clinical N2 disease to pathologic N0 disease was significantly higher in the ICI group (68.6%, 42.9%, and 31.7% for the ICI, EGFR-TKI, and chemotherapy groups, respectively, P=0.012).
Table 3
| Variables | ICI plus chemotherapy (N=42) | EGFR-TKI (N=54) | Chemotherapy alone (N=98) | P value |
|---|---|---|---|---|
| Pathologic response, n (%) | <0.001 | |||
| Pathologic complete response | 17 (40.5) | 6 (11.1) | 6 (6.1) | |
| Major pathologic response | 30 (71.4) | 11 (20.4) | 14 (14.3) | |
| <90% response | 12 (28.6) | 43 (79.6) | 84 (85.7) | |
| yp TNM stage, n (%) | 0.002 | |||
| I | 29 (69.0) | 27 (50.0) | 33 (33.7) | |
| II | 3 (7.1) | 6 (11.1) | 23 (23.5) | |
| III | 10 (23.8) | 21 (39.9) | 42 (42.8) | |
| Pathological tumor size (cm), mean ± SD | 2.9±1.7 | 2.6±1.0 | 3.4±1.7 | 0.018 |
| Downstaging of nodal status for N2 disease , n (%) | 0.012 | |||
| N2 to pN0 | 24 (68.6) | 15 (42.9) | 19 (31.7) | |
| N2 to pN1 | 1 (2.8) | 4 (11.4) | 9 (15.0) | |
| N2 to pN2 | 10 (28.6) | 16 (45.7) | 32 (53.3) | |
ICI, immune checkpoint inhibitor; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitors; TNM, tumor, node, metastasis; SD, standard deviation.
Discussion
Incorporating ICI plus chemotherapy into the management of early-stage resected NSCLC is now an area of active investigation (7,10,11). However, concerns about the feasibility and safety of surgical resection after neoadjuvant ICI still exist as mediastinal or hilar inflammation/fibrosis might develop in response to treatment. In this study, we reviewed 42 patients who received neoadjuvant ICI plus chemotherapy followed by surgical resection and found that neoadjuvant ICI was associated with a higher rate of achieving MPR and a similar postoperative mortality and morbidity compared with neoadjuvant EGFR-TKI and neoadjuvant chemotherapy alone in resectable NSCLC. To the best of our knowledge, this is the first study to comprehensively evaluate perioperative outcomes after neoadjuvant ICI plus chemotherapy and compare it to other neoadjuvant regimens.
Neoadjuvant ICI in resected NSCLC was first reported in 2018. Two doses of nivolumab were associated with 45% of cases achieving MPR and 10% of cases achieving pCR (7). Recent phase II trials also have shown that 57–74% of patients experience MPR after neoadjuvant ICI, and without treatment-related surgical delays (10,11). In this study, the proportion of patients achieving MPR was 71.4% in the neoadjuvant ICI plus chemotherapy group, which was significantly higher than in the neoadjuvant EGFR-TKI and neoadjuvant chemotherapy alone groups, and no treatment-related surgical delay was observed. The effectiveness and safety of neoadjuvant ICI plus chemotherapy have been corroborated by the CheckMate 816 study (12). The perioperative outcomes after neoadjuvant ICI plus chemotherapy have not been evaluated comprehensively and compared with standard neoadjuvant chemotherapy alone, despite the emergence of numerous clinical trials using neoadjuvant ICI (25).
Yang et al. first evaluated surgical outcomes after neoadjuvant ipilimumab plus chemotherapy for stage II-IIIA NSCLC based on a phase II trial (TOP1201), demonstrating the safety and feasibility of surgical resection (26). Bott et al. reported perioperative outcomes after neoadjuvant nivolumab based on a randomized trial and concluded that the surgery, while challenging, could be accomplished safely (13). Minimally invasive resections have been shown to be performed safely following monotherapy of ICI. However, more than half of VATS or robotic cases were converted to thoracotomy often because of hilar inflammation and fibrosis. In the current study, all patients were completely resected and most of the tumors (88.1%) were resected via VATS after neoadjuvant ICI plus chemotherapy, which is higher than previous reports (26,27). There was only one patient in the ICI plus chemotherapy group converted to thoracotomy due to severe hilar fibrosis. It is worth noting that patients in the chemotherapy alone group had a larger tumor size before surgery, which can explain a lower use of VATS resections and thereby a higher proportion of postoperative pneumonia associated with thoracotomies. Ultimately, this study showed that neoadjuvant ICI plus chemotherapy was not associated with unexpected perioperative mortality or postoperative complications compared with neoadjuvant EGFR-TKI and neoadjuvant chemotherapy alone.
Thomas et al. reported that neoadjuvant chemotherapy increased treatment-related mortality compared to patients without neoadjuvant chemotherapy, especially in patients undergoing a pneumonectomy (14% vs. 6%), which led to the absence of a progression-free survival benefit (28). In this study, 7.3% of patients underwent pneumonectomy after neoadjuvant treatment, and the associated perioperative mortality was 7.1%. One patient in the chemotherapy group experienced food aspiration, leading to severe pneumonia and ongoing hypoxemia and eventually, death. In this study, neoadjuvant treatment was not associated with a higher rate of perioperative mortality after pneumonectomy compared with those without neoadjuvant treatment, which is consistent with the findings of a previous study (29).
Sleeve lobectomy is an optional surgical procedure to improve postoperative quality of life compared with pneumonectomy for centrally located NSCLC, but it remains challenging, especially via VATS (30). Yang et al. reported that sleeve lobectomy achieved superior perioperative outcomes and equivalent oncological efficacies compared with pneumonectomy (31). In this study, 76 patients with centrally located NSCLC received neoadjuvant treatment, and 41 patients eventually were resected by sleeve lobectomy. Additionally, sleeve lobectomy was associated with a shorter hospital stay and similar postoperative complications compared with pneumonectomy (Table S1). Our previous study also found double sleeve lobectomy after neoadjuvant chemotherapy is safe and no perioperative mortality in patients with centrally-located NSCLC than those without neoadjuvant chemotherapy (32). In the current study, one (7.1%) sleeve lobectomy patient in the ICI plus chemotherapy group experienced bronchopleural fistula and eventually die. We believe that the safety of sleeve lobectomy after ICI plus chemotherapy need more evidence to verify.
This study had several limitations. First, the smaller sample size for the neoadjuvant ICI patients may have limited the statistical power, and the effect of the surgical extent and surgical approach on perioperative outcomes were not available. Second, the results were derived from retrospective study, using a single-center database, with inevitable selection bias. Some clinicopathologic factors, such as gender, smoking history, histologic subtype, and treatment cycles may not be well balanced. Prospective multicenter clinical trials are required to provide more evidence. Third, the baseline characteristics among the three groups were imbalanced. The results of additional forthcoming prospective studies on this topic should address this issue.
Conclusions
Surgical resection after neoadjuvant ICI plus chemotherapy appeared to be safe and feasible without unexpected perioperative complications and mortalities for patients with resected NSCLC compared with neoadjuvant EGFR-TKI and neoadjuvant chemotherapy alone, and our experience suggested that most of these procedures could be accomplished with VATS approach. Additional prospective randomized studies using larger patient cohorts are necessary to validate our findings.
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group. This paper was presented at the 101st Annual Meeting of the American Association for Thoracic Surgery, A Virtual Learning Experience, April 30–May 2, 2021.
Funding: This study was supported by the Shanghai Hospital Development Center (Nos. SHDC2020CR1021B, SHDC22021217, and SHDC22021310-A), Shanghai Science and Technology Commission (20DZ2253700), the National Natural Science Foundation of China (NSFC 9195910169), and Shanghai Municipal Health Commission (202040322).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-476/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-476/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-476/coif). FG serves in Ad Hoc Advisory Boards/Consultations (last 3 years) to Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, BMS, MSD, Novartis, Merck, Otsuka, Novartis, Takeda. FG also received Research Funding (last 3 years) from AstraZeneca, BMS, MSD and received honoraria (last 3 years) from Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, AMGEN, Celgene, BMS, MSD. AK received support from Roche Genentech and Medtronic and serves as Advisory Board for neoadjuvant immunotherapy trials for Roche Genentech. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Shanghai Pulmonary Hospital (No. L21-224), and written informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Stupp R, Mayer M, Kann R, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol 2009;10:785-93. [Crossref] [PubMed]
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [Crossref] [PubMed]
- Buddharaju LNR, Ganti AK. Immunotherapy in lung cancer: the chemotherapy conundrum. Chin Clin Oncol 2020;9:59. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Liu J, Blake SJ, Yong MC, et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov 2016;6:1382-99. [Crossref] [PubMed]
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463-9. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. [Crossref] [PubMed]
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. [Crossref] [PubMed]
- Zhuang X, Zhao C, Li J, et al. Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med 2019;8:2858-66. [Crossref] [PubMed]
- Kawaguchi T, Koh Y, Ando M, et al. Prospective Analysis of Oncogenic Driver Mutations and Environmental Factors: Japan Molecular Epidemiology for Lung Cancer Study. J Clin Oncol 2016;34:2247-57. [Crossref] [PubMed]
- Li S, Choi YL, Gong Z, et al. Comprehensive Characterization of Oncogenic Drivers in Asian Lung Adenocarcinoma. J Thorac Oncol 2016;11:2129-40. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Zhong WZ, Chen KN, Chen C, et al. Erlotinib Versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. J Clin Oncol 2019;37:2235-45. [Crossref] [PubMed]
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Xie D, Wang H, Fei K, et al. Single-port video-assisted thoracic surgery in 1063 cases: a single-institution experience†. Eur J Cardiothorac Surg 2016;49:i31-6. [Crossref] [PubMed]
- Al Sawalhi S, Ding J, Vannucci J, et al. Perioperative risk factors for atrial fibrillation (AF) in patients underwent uniportal video-assisted thoracoscopic (VATS) pneumonectomy versus open thoracotomy: single center experience. Gen Thorac Cardiovasc Surg 2021;69:487-96. [Crossref] [PubMed]
- Xie D, Deng J, Gonzalez-Rivas D, et al. Comparison of video-assisted thoracoscopic surgery with thoracotomy in bronchial sleeve lobectomy for centrally located non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;161:403-413.e2. [Crossref] [PubMed]
- Chen J, Soultanis KM, Sun F, et al. Outcomes of sleeve lobectomy versus pneumonectomy: A propensity score-matched study. J Thorac Cardiovasc Surg 2021;162:1619-1628.e4. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Huynh C, Walsh LA, Spicer JD. Surgery after neoadjuvant immunotherapy in patients with resectable non-small cell lung cancer. Transl Lung Cancer Res 2021;10:563-80. [Crossref] [PubMed]
- Yang CJ, McSherry F, Mayne NR, et al. Surgical Outcomes After Neoadjuvant Chemotherapy and Ipilimumab for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:924-9. [Crossref] [PubMed]
- Brunelli A, Rocco G, Szanto Z, et al. Morbidity and mortality of lobectomy or pneumonectomy after neoadjuvant treatment: an analysis from the ESTS database. Eur J Cardiothorac Surg 2020;57:740-6. [Crossref] [PubMed]
- Thomas M, Rübe C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48. [Crossref] [PubMed]
- Hennon MW, Kumar A, Devisetty H, et al. Minimally Invasive Approaches Do Not Compromise Outcomes for Pneumonectomy: A Comparison Using the National Cancer Database. J Thorac Oncol 2019;14:107-14. [Crossref] [PubMed]
- Wang X, Jiang S, You X, et al. Extended Sleeve Lobectomy is an Alternative for Centrally Located Lung Cancer With Superior Short- and Long-term Outcomes. Clin Lung Cancer 2021;22:e621-8. [Crossref] [PubMed]
- Yang M, Zhong Y, Deng J, et al. Comparison of Bronchial Sleeve Lobectomy With Pulmonary Arterioplasty Versus Pneumonectomy. Ann Thorac Surg 2022;113:934-41. [Crossref] [PubMed]
- Bao Y, Jiang C, Wan Z, et al. Feasibility of double sleeve lobectomy after neoadjuvant chemotherapy in patients with non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2022; Epub ahead of print. [Crossref] [PubMed]


