
Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis
Introduction
In recent years, treatment algorithms in advanced non-small cell lung cancer (NSCLC) have dramatically evolved (1). Immune checkpoint inhibitors (ICIs) directed against cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1) or programmed death ligand 1 (PD-L1) have contributed to the exciting progress in the treatment of locally advanced and/or metastatic NSCLC as a result of their impressive clinical survival benefit (2-5). ICIs can restore or enhance the anti-tumor immune response by interrupting the signaling pathway of T-cell inhibition to help to positively regulate T-cell activity (6). A large proportion of patients with NSCLC have experienced long-term survival benefit from immunotherapy, though they may eventually consider requiring discontinuation of ICIs treatment due to disease progression, immune-related adverse events (irAEs) or completion of a fixed duration course of ICIs treatment without progression. Against this background, evidence is mounting that ICIs retreatment could be a potential option considering the dynamic nature of the immune response and long-term benefit of ICIs. When ICIs are discontinued because of progressive disease, there are no established strategies to overcome acquired resistance. The clinical benefit of chemotherapy or targeted therapy in combination with immunotherapy has been presented in clinical trials (7). Chemotherapy kills tumor cells through cytotoxicity directly, hamper the immune escape of tumor cell, alter tumor microenvironment to make it easier to be recognized by the immune system, while angiogenesis inhibitors can inhibit the production of new blood vessels around tumors to affect the growth and metastasis of malignant tumor. Otherwise, local therapy of primary or metastasis lesion in oligoprogression or oligometastasis has been shown to prolong the benefit from ICIs (7). Patients with NSCLC may experience irAEs resulting from augmented immune response and unbalance of the immune system. Although most irAEs could be resolved after discontinuation of ICIs and management with steroids or immunosuppressive agents, whether they could be retreated with ICIs remains under debate considering the occurrence and recurrence of irAEs. As for those who progress after a fixed duration of ICIs treatment, emerging data suggests that they could experience clinical benefits from ICIs retreatment at disease relapse (8,9), while it is still difficult to draw a definitive conclusion.
ICIs retreatment has been applied in advanced melanoma patients, who are allowed to be retreated with the same anti-CTLA-4 agent or to receive a sequential administration of anti-CTLA-4 and anti-PD-1/PD-L1 (10-13). Ravi et al. also reported that ICIs retreatment in patients with metastatic renal cell carcinoma may be safe and reasonably efficacious with an objective response rate (ORR) of 23% and an incidence of Grade 3 or higher irAEs of 16% (14). Gul et al. (15) focused on salvage therapy with Nivolumab plus ipilimumab in patients with mRCC previously treated with PD-1/PD-L1 inhibitors, which showed an ORR of 20%. Previous studies focused on safety and efficacy of ICIs retreatment were mostly cohort studies. Zhang et al. reported a subgroup analysis for tumor type which showed the ORR of anti-PD-1/PD-L1 antibodies for NSCLC were 21% (16). Therefore, we conducted a systematic review and meta-analysis to evaluate the safety and efficacy of ICIs retreatment after prior ICIs treatment in patients with NSCLC to clarify the efficacy-safety balance of ICIs retreatment. Furthermore, it is worthwhile that ICIs retreatment should be evaluated based on different reasons for termination of initial ICIs treatment considering the heterogeneity among patients. We present the following article in accordance with the PRISMA reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-140/rc).
Methods
Search strategy
We searched PubMed, Web of Science, Embase and Cochrane library to identity relevant studies. The last search date was November 21st, 2021. The following retrieval terms were combined with Boolean operators (AND, OR, NOT): (Non-small Cell Lung Cancer OR Non-small Cell Lung Carcinoma OR NSCLC) AND (immune checkpoint inhibitors OR PD-1 OR PD-L1 OR CTLA-4) AND (retreatment OR rechallenge OR resumption OR re-administration OR restart OR reinduction). Reference of involved studies and previously published systematic review and meta-analysis were manually checked one by one in order to avoid missing any other relevant articles.
Inclusion and exclusion criteria
Studies which tally with the following criteria are eligible to be included: (I) enrolled at least 10 adult patients with NSCLC, (II) patients were treated with ICIs previously and discontinued the treatment because of progression, irAEs or completion of a fixed course, (III) patients were retreated with ICIs after a period of break, (IV) the stage of NSCLC was not limited to stage III and IV. Exclusion criteria included: (I) animal trials, (II) case report, editorials, abstract of conference without detailed data and ongoing clinical trials without published data, (III) no detailed information of clinical outcomes of prior treatment and retreatment, (IV) patients concurrently retreated with ICIs and other treatment. Two independent researchers reviewed all potentially eligible articles filtered by above criteria in order to solve discrepancy and a third researcher could be consulted with when necessary.
Data extraction and endpoints
Two researchers checked the full text of all eligible studies and extracted data from them independently. Discrepancies arising from the selection of eligible studies and extraction of data were resolved by reaching a consensus with the third researcher. Detailed study characteristics extracted from these studies included author, publication year, study design, enrollment, type of initial immunotherapy and ICIs retreatment, reason for discontinuation of prior treatment, incidence of initial and retreated irAEs, as well as ORR and disease control rate (DCR) of initial treatment and retreatment. The severity of irAEs was divided into grade 1 to 5 according to the Common Terminology Criteria for Adverse Events (CTCAE). Grade ≥3 was defined as high-grade irAEs. ORR refers to the rate of patients who showed a complete or partial response, while DCR implies the rate of patients who had a complete or partial response or stable disease. Methodological quality of all involved studies was assessed according to the Newcastle-Ottawa Scale (NOS) criteria for observational and retrospective studies, which ranging from 0 (poor quality) to 9 (optimal quality).
Definition of retreatment, rechallenge and resumption
We defined ‘Retreatment’ as re-administration with agents aimed at blocking the immune checkpoint for patients who discontinued initial ICIs treatment for any reason after a period of break. ICIs rechallenge implies retreatment applied to patients who progressed during treatment or within 12 weeks of termination of immunotherapy. ICIs resumption aims at those who previously discontinued immunotherapy because of irAEs or progression after a fixed course of ICIs treatment in the absence of disease progression.
Statistical analysis
Our study employed Review Manager software (version 5.4.1) to perform statistical analysis and draw forest plots. Summary statistics were presented as total and percentage for categorical variables. Synthesis of all-grade or high-grade irAE, ORR and DCR of initial ICIs treatment and ICIs retreatment were calculated as pooled rates by using pooled odds ratios (OR) with 95% confidence intervals (95% CI) and generic inverse variance method. As to ICIs rechallenge and ICIs resumption, the same calculation method was performed. Subgroup analysis was conducted using random-effects model based on the cause of previous ICIs discontinuation and type of ICIs retreatment. Considering that most included studies were retrospective which may cause heterogeneity significantly, random-effects model with the Mantel-Haenszel model was adopted for analysis. Heterogeneity was assessed by I-squared test (values <25% indicate low heterogeneity; 25–75%, moderate heterogeneity; and >75%, considerable heterogeneity). Sensitivity analyses were performed by excluding each study to explore the stability of those pooled estimates. Results were regarded statistically significant when P value was less than 0.05.
Results
Study characteristics
We identified 240 articles through database search and 6 additional articles were retrieved by searching the reference of the included studies and from previous systematic reviews and meta-analysis. After removing duplicates and ineligible articles and screening titles and abstracts, 18 studies were ultimately enrolled for qualitative and quantitative pooled analysis (Figure 1). Table 1 summarizes the characteristics of 18 included studies. Two studies were prospective, while the others were retrospective.
Table 1
| Author | Initial ICIs | Initial irAEs | Reason for interruption | ICIs Retreatment | Retreated irAEs | mOS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | ORR | DCR | mPFS(m) | All-grade irAEs | High-grade irAEs | Type | ORR | DCR | mPFS(m) | All-grade irAEs | High-grade irAEs | |||||
| Brahmer 2020 (8) | Anti PD-1 | – | – | – | – | – | PD | Anti PD-1 | 4/12 | 10/12 | – | – | – | – | ||
| Fujisaki 2021 (17) | Anti PD-1 | 14/38 | 22/38 | 11.3 | – | 15/38 | irAE | Anti PD-1 | 10/14 | 14/14 | 15.3 | – | 4/14 | – | ||
| Fujita 2018 (18) | Anti PD-1 | 7/12 | 9/12 | 6.3 | – | – | PD | Anti PD-1 | 1/12 | 5/12 | 3.1 | – | – | – | ||
| Fujita 2019 (19) | Anti PD-1 | 7/18 | 11/18 | – | – | 9/18 (G≥2) | PD | Anti PD-L1 | 0 | 7/18 | 2.9 | – | 15/18 (G≥2) | – | ||
| Fujita 2020 (20) | Anti PD-L1 | 0 | 5/15 | – | – | 14/15 (G≥2) | PD | Anti PD-1 | 0 | 4/15 | 2.3 | – | 9/15 (G≥2) | – | ||
| Furuya 2021 (21) | Anti PD-1 | 8/38 | 24/38 | 3.4 | 56/152 | – | irAE, PD | Anti PD-L1 | 1/38 | 13/38 | 1.9 | 9/38 | – | – | ||
| Giaj Levra 2020 (22) | Anti PD-1 | – | – | – | – | – | PD | Anti PD-1 | – | – | – | – | – | 14.8 | ||
| Gobbini 2020 (23) | Anti PD-(L)1 | 71/144 | 109/144 | 13 | – | 27/144 | PD, irAE, clinical decision | Anti PD-(L)1 | 23/144 | 68/144 | 4.4 | – | 9/144 | 18 | ||
| Herbst 2020 (9) | Anti PD-1 | 13/14 | 13/14 | – | – | – | Clinical decision | Anti PD-1 | 6/14 | 11/14 | – | – | – | – | ||
| Katayama 2019 (24) | Anti PD-(L)1 | 12/35 | 24/35 | 4 | – | – | PD | Anti PD-(L)1 | 1/35 | 15/35 | 2.7 | – | – | 7.5 | ||
| Kitagawa 2020 (25) | Anti PD-(L)1 | 6/17 | 15/17 | – | 10/17 | 3/17 | PD, irAE | Anti PD-(L)1 | 1/17 | 10/17 | 4.0 | 5/17 | 2/17 | 31 | ||
| Koyauchi 2020 (26) | Anti PD-1 | 35/79 | 57/79 | – | 79/592 | 30/592 | irAE | Anti PD-1 | 8/16 | 14/16 | – | 5/16 | 0 | – | ||
| Mouri 2019 (27) | Anti PD-1 | 13/21 | 21/21 | – | 49/187 | 12/187 | irAE | Anti PD-1 | 3/21 | 18/21 | 14.4 | 15/21 | 1/21 | – | ||
| Niki 2018 (28) | Anti PD-1 | 5/11 | 7/11 | 4.9 | 5/11 | 0 | PD | Anti PD-1 | 3/11 | 5/11 | 2.7 | 5/11 | 0 | – | ||
| Santini 2018 (29) | Anti PD-(L)1 or anti PD-(L)1 plus CTLA-4 | 30/68 | – | – | 68/482 | 33/482 | irAE | Anti PD-L1 | 18/38 | 31/38 | – | 20/38 | 8/38 | – | ||
| Sheth 2020 (30) | Anti PD-L1 | – | – | – | – | – | Clinical decision | Anti PD-L1 | 3/21 | 11/21 | – | – | – | – | ||
| Takahama 2018 (31) | Anti PD-1 or anti PD-L1 | 5/10 | 7/10 | – | – | – | PD | ICIs | 0 | 3/10 | – | – | – | – | ||
| Watanabe 2019 (32) | Anti PD-(L)1 | 3/14 | 8/14 | 3.7 | 9/14 | – | PD | Anti PD-1 | 1/14 | 3/14 | 1.6 | 5/14 | 0 | 6.5 | ||
High-grade irAEs was defined as grade ≥3. Clinical decision referred to patients who had completed a fixed course of ICIs. PD-1, programmed death-1; PD-L1, programmed cell death-ligand 1; CTLA-4, cytotoxic T-lymphocyte antigen-4; ICIs, immune checkpoint inhibitors; ORR, objective response rate; DCR, disease control rate; irAEs, immune-related adverse events; PFS, progression-free survival; OS, overall survival; PD, progression disease.
Efficacy
Sixteen studies (8,9,17-21,23-29,31,32) were enrolled in the analysis of efficacy of ICIs retreatment. The pooled ORR and DCR of ICIs retreatment were respectively 20% and 54% (Table 2). ICIs retreatment was associated with a decrease in ORR and DCR compared to prior ICIs treatment (ORR: OR, 0.29; 95% CI: 0.14–0.63; P=0.002; I2=74%); (DCR: OR, 0.53; 95% CI: 0.28–0.99; P=0.05; I2=66%) (Figure S1).
Table 2
| Reasons for discontinuation of prior ICIs | ORR | DCR | All-grade irAEs | High-grade irAEs |
|---|---|---|---|---|
| Retreatment (overall) | 20% | 54% | 41% | 13% |
| Rechallenge after PD | 8% | 39% | – | – |
| Resumption after irAEs and clinical decision | 34% | 71% | – | – |
Grade ≥3 was defined as high-grade irAEs. ORR, objective response rate; DCR, disease control rate; PD, progression disease; irAEs, immune-related "adverse" events; ICI, immune checkpoint inhibitor.
We further performed subgroup analysis according to the reasons for discontinuation of prior ICIs treatment. The pooled ORR and DCR of ICIs rechallenge were 8% and 39% (Table 2) respectively which were applied for patients who interrupted prior ICIs treatment because of progression. ICIs rechallenge showed a lower ORR and DCR compared with initial ICIs treatment (ORR: OR, 0.13; 95% CI: 0.06–0.29; P<0.05; I2=0%) (DCR: OR, 0.33; 95% CI: 0.21–0.53; P<0.05; I2=0%). In contrast, the pooled ORR and DCR of ICIs resumption were 34% and 71% (Table 2) in studies where patients discontinued the initial treatment due to irAEs or completion of a fixed course, showing no significant difference in ORR and DCR compared with initial immunotherapy (ORR: OR, 0.67; 95% CI: 0.24–1.87; P>0.05; I2=74%) (DCR: OR, 1.81; 95% CI: 0.38–8.69; P>0.05; I2=69%) (Figure 2).
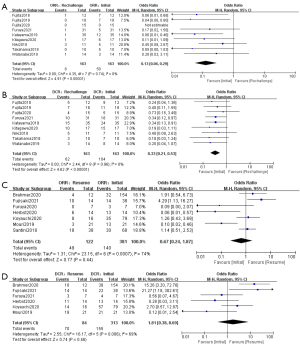
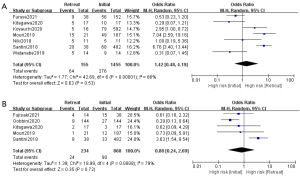
Further analysis revealed that the efficacy of retreatment might vary from ICI to ICI. Patients who were retreated with the same type of ICI as before showed no difference for ORR and DCR (ORR: OR, 0.37; 95% CI: 0.09–1.52; P>0.05; I2=78%) (DCR: OR, 0.76; 95% CI: 0.20–2.92; P>0.05; I2=60%). As to those retreated with different ICIs, such as switching from anti-PD-1 to anti-PD-L1, displayed a decrease in ORR and DCR in contrast to initial treatment (ORR: OR, 0.09; 95% CI: 0.02–0.34; P<0.05; I2=0%) (DCR: OR, 0.35; 95% CI, 0.18–0.67; P<0.05; I2=0%) (Figure S2).
Safety
Nine studies (17,21,23,25-29,32) were involved in the analysis of safety. The occurrence rate of all-grade and high-grade irAEs were 41% and 13% separately (Table 2). The incidence of all-grade and high-grade irAEs were not significantly different between initial ICIs treatment and ICIs retreatment (all-grade: OR, 1.42; 95% CI: 0.48–4.19; P=0.53; I2=86%; high-grade: OR, 0.80; 95% CI: 0.24–2.69; P=0.72; I2=79%) (Figure 3).
Discussion
In our study, the ORR and DCR of ICIs retreatment in patients with NSCLC were respectively 20% and 54%, which cohered with a meta-analysis showing a median ORR of 21.8% by evaluating patients with solid tumors (33). Moreover, the incidence of all-grade and high-grade irAEs in retreated patients was 41% and 13% separately, which is comparable to what reported for first immunotherapy (34,35). Taken together, our discoveries seem to indicate that retreatment with ICIs could be a feasible and effective therapeutic option after cessation of prior ICIs treatment for a variety of reasons.
It is worth noting that we found the discrepancy in efficacy of ICIs retreatment in patients with NSCLC who discontinued prior ICIs for disease progression, irAEs or completion of a fixed course. Patients with NSCLC who undergo immunotherapy retreatment may represent a heterogeneous population. While those previous studies focused only on the total efficacy and safety of ICIs retreatment without any consideration for heterogeneity among patients or tumor type, our study is the first meta-analysis to define what is ICIs rechallenge and ICIs resumption according to the reason for the discontinuation of prior ICI treatment in patients with NSCLC rather than ambiguously refer as ICIs rechallenge in general regardless of heterogeneity. Meanwhile, we try to explore the mechanism for difference in efficacy in ICIs rechallenge and resumption based on previous studies.
The pooled ORR and DCR of ICIs rechallenge which were applied to patients who progressed during prior ICIs were 8% and 39%. ICIs rechallenge was associated with a decrease in ORR and DCR compared with initial treatment. Based on above observation, it indicated that the clinical benefit of ICIs rechallenge was limited to patients with NSCLC. Primary, adaptive, and acquired resistance to immunotherapy to some degrees could explain the poor response to subsequent ICIs rechallenge. In clinical scenarios where a tumor does not respond to immunotherapy or is recognized by the immune system, but it protects itself by adapting to the immune attack, patients do not respond to ICIs (36). However, in these clinical scenarios, patients may have already showed poor response to initial ICIs treatment what ICIs rechallenge should take into consideration. When it comes to patients who responded for a period and then progressed, the potential mechanisms of progression include loss of T cell function, lack of T cell recognition by down-regulation of tumor antigen presentation and development of escape mutation variants in the lung cancer. As a result, the high activity and broad use of prior ICIs might exhaust the host immune status and lead to the poor response to ICIs rechallenge (36). Although the ORR and DCR of ICIs rechallenged patients decreased significantly compared with that of first ICIs treatment, nearly forty percent of patients can still regain control of disease, what was comparable to the survival data of third-line standard chemotherapy for advanced NSCLC and mono-chemotherapy after ICIs progression (37,38). Several studies are under way to address different strategies of rechallenge, such as ‘Re-challenge Pembrolizumab Study as a second or further line in patients with advanced NSCLC’ (NCT03526887), ‘Ipilimumab and Nivolumab in patients with anti-PD-1-axis therapy-resistant advanced NSCLC’ (NCT03262779), ‘Single agent chemotherapy +/− Nivolumab in patients with advanced squamous or non-squamous NSCLC with primary resistance to prior PD-1 or PD-L1 inhibitor’ (NCT03041181) and HUDSON (NCT03334617), what may help maximize the efficacy of ICIs rechallenge by selecting the most appropriate patients and treatment and understanding the mechanism underlying resistance to immunotherapy.
By contrast, the pooled ORR and DCR of ICIs resumption were 34% and 71%. No significant difference for ORR and DCR were noted between resumption and initial treatment, which implies similar efficacy. Based on these data, it is feasible to consider ICIs resumption as a subsequent treatment. While some retrospective studies which reported progression-free survival (PFS) and overall survival (OS) suggest that the prognostic impact of discontinuation cohort was like that of resumption cohort in term of long-survival benefit (39,40). It seems that ICIs resumption did not confer any greater long-term survival benefit than drug withdrawal, which might expose a risk of recurrence or occurrence of irAEs. Further study is warranted to compare the survival benefit of resumption and discontinuation in large sample size.
ICIs resumption can be applied in two clinical scenarios. Emerging studies suggest that patients with advanced NSCLC who progressed after finishing a fixed course of ICIs treatment could experience clinical benefit from ICIs resumption whether the fixed course in one or two years (9,30,41). In this situation, ICIs resumption may reboot the expansion of memory T cell against tumor to help restore sensitivity to resumption so as to achieve disease control and even long-term benefits. Sheth et al. (30) found that the greater benefit was noted in individuals who had a treatment-free interval since prior durvalumab ≥6 months compared with <6 months, which indicated that the duration between prior ICIs and resumption might be related to the efficacy of ICIs resumption.
In another situation, our data suggest that patients who discontinued first ICIs due to irAEs could be target population for ICIs resumption. The decision of resumption may hinge on the type and severity of irAEs patients confronted with during prior ICIs. Most guidelines recommended that Grade 3 irAEs should be treated with high-dose intravenous steroids (42,43). In case of Grade 4 or higher irAEs, immunotherapy will be terminated permanently which derived from informal expert consensus or clinical experience (44-46). However, Haratani et al. (47) reported that the ORR was significantly higher in patients with irAEs than those without and patients who developed grade 3 or higher irAEs were significantly associated with increased PFS, which shared a similar conclusion with a retrospective review of 290 patients with advanced NSCLC treated on an immunotherapy-base clinical trial (48). On this basis, it is controversial whether ICIs resumption is a promising therapeutic option after discontinuation of prior treatment due to serious irAEs. Park et al. performed a meta-analysis to explore the recurrence of irAEs in patients who resumed with ICIs after discontinuation of prior ICIs for irAEs. The incidence of any grade, severe grade (grade 3 or 4) or steroid-requiring irAEs were 47%, 13.2% and 26%, which were comparable with historical incidences of irAE in treatment-naive patients. The risk of severe irAEs was lower in the resumption setting compared with the incidence of irAE in the previous treatment period. Moreover, subgroup analysis showed the risk of severe irAEs was driven mostly by the subgroup who received combination therapy as the initial regimen. To further explore the incidence of irAEs in patients with NSCLC who discontinued prior ICIs treatment for irAEs, our study shows that the occurrence and recurrence of irAEs is 41.5% (OR, 0.71; 95% CI: 0.16–0.57; P=0.48) and 27.5% (OR, 0.71; 95% CI: 0.08–0.20; P<0.05) which is comparable to Park’s discovery and of acceptive safety. However, the information is too limited to draw a definitive conclusion about common types of irAE in ICIs resumption or more. Koyauchi et al. (26)focused on anti-PD-1 antibody-related pneumonitis, which showed an incidence of recurrent pneumonitis of 31.2% in their resumed cases. Taking the efficacy and safety of ICIs resumption into account, clinical physicians should carefully evaluate on a case-by-case basis and weigh pros and cons before deciding on whether ICIs should be resumed in patients who terminated treatment for irAEs.
Our analysis also found that no significant difference was noted for ORR and DCR in patients retreated with same type of ICI as initial ICI, while retreatment with different type was factor associated with a lower ORR and DCR. Similarly, two studies in melanoma reported that response to the first anti-PD-1 antibody was predictive of efficacy of retreatment with a second anti-PD-1 antibody. Among patients who switched from anti-PD-1/PD-L1 to anti-PD-L1/PD-1, retreatment was of limited benefit. The phenomenon mainly stems from the fact that most patients involved in this analysis received anti-PD-L1 as a later-line therapeutic regimen after multiple anti-cancer treatment including cytotoxic chemotherapy and radiotherapy what might cause a poor performance and physically exhausted status to depress immune response. Fujita et.al retrospectively examined patients who switched from anti-PD-1/PD-L1 to anti-PD-L1/PD-1 (19,20). A lager number of these patients received atezolizumab as a second- or later-line regimen in the two studies and three patients even amounting to triple ICIs rechallenge. The prolong use of ICIs might exhaust the host immune status to respond to ICIs rechallenge poorly. Another possible explanation for this outcome is that the vast majority of patients who retreated with different type of ICIs terminated initial treatment because of progression. Among people who retreated with different types of ICIs, almost 93% discontinued prior ICIs treatment due to progressive disease, while only 44% in the subgroup with the same type of ICIs. Noteworthy, Bernard-Tessier et al. (48) evaluated the efficacy of retreatment with the same ICI in several types of tumors and the results indicated that the clinical benefit from this was limited. Which regimen of ICIs retreatment to choose is still controversial and there is clearly a need for multicenter, large-scale trials to aid ICIs retreatment in the future regarding the regimen of retreatment.
With further research, ICIs are being more commonly giving in combination with chemotherapy for advanced NSCLC as first-line regimen. It has been proved that the combination of immunotherapy and chemotherapy could yield significant clinical benefits in overall survival and progression-free survival (49-51). Compared with immunotherapy alone, after combined with chemotherapy, the clinical benefit is significantly improved. Considering the improved efficacy in ICI plus chemotherapy as first-line treatment, it may be feasible to add chemotherapy to ICIs retreatment at the same time. All patients included in our analysis were retreated with ICIs alone, even though some patients received chemotherapy or radiotherapy between ICI treatment and retreatment. The efficacy and safety of ICI retreatment in combination with chemotherapy concurrently remains unknown which deserves further exploration in the future.
Our analysis has several important limitations that require consideration when interpreting the results. First, most involved studies are non-randomized, retrospective what may result in selection bias and raise concerns for the quality of evidence. The timing of retreatment and regimens were chosen by clinical physicians and therefore not standardized between patients. Second, we performed meta-analysis for ORR and DCR to assess efficacy of ICIs retreatment instead of PFS and OS which might be more convincing. Third, the incidence of all-grade and high-grade irAEs were not assessed according to the reason for discontinuation since these data were not reported systematically in the involved studies. Finally, further statistical analysis to explore predictive markers associated with the efficacy of ICIs retreatment is hard to perform due to the insufficient data extracted from the recruited studies.
Conclusions
Taken all together, ICIs retreatment could constitute a feasible therapeutic option in selected NSCLC patients who have ceased the previous ICIs treatment for different reasons, especially in those who discontinued for irAEs or finished given course of treatment. However, ICIs retreatment should be mulled over on a case-by-case basis in consideration of possible factors linked to the efficacy, such as reason for termination, performance status, interval treatment regimens, the type of ICI in retreatment and the type and severity of irAEs. More large-scale prospective studies are warranted to confirm our discoveries and explore the biomarkers that predict the efficacy and safety of ICIs retreatment in patients with NSCLC. Moreover, we should pay more attention to the topic on retreatment concurrently with ICIs and other treatment such as chemotherapy, radiotherapy and targeted therapy in the future.
Acknowledgments
We would like to thank all the reviewers who made contribution to this article.
Funding: This study was supported by the National Natural Science Foundation of China (No. 82172728), the Natural Science Foundation of Jiangsu Province (No. BE2019718) and the Social Development Foundation of China (No. BE2019719).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-140/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-140/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-140/coif). YS serves as an Editor-in-Chief of Translational Lung Cancer Research from September 2014 to August 2022. TL serves as an unpaid Associate Editor-in-Chief of Translational Lung Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Toschi L, Rossi S, Finocchiaro G, et al. Non-small cell lung cancer treatment (r)evolution: ten years of advances and more to come. Ecancermedicalscience 2017;11:787. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-82. [Crossref] [PubMed]
- Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol 2020;21:e463-76. [Crossref] [PubMed]
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. KEYNOTE-024 5-year OS update: First-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50%. Ann Oncol 2020;31:S1142-215. [Crossref]
- Herbst RS, Garon EB, Kim DW, et al. Long-Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death-Ligand 1‒Positive, Advanced Non‒Small-Cell Lung Cancer in the KEYNOTE-010 Study. J Clin Oncol 2020;38:1580-90. [Crossref] [PubMed]
- Larkin J, Minor D, D'Angelo S, et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator's Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol 2018;36:383-90. [Crossref] [PubMed]
- Weber JS, Gibney G, Sullivan RJ, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 2016;17:943-55. [Crossref] [PubMed]
- Chiarion-Sileni V, Pigozzo J, Ascierto PA, et al. Ipilimumab retreatment in patients with pretreated advanced melanoma: the expanded access programme in Italy. Br J Cancer 2014;110:1721-6. [Crossref] [PubMed]
- Shreders A, Joseph R, Peng C, et al. Prolonged Benefit from Ipilimumab Correlates with Improved Outcomes from Subsequent Pembrolizumab. Cancer Immunol Res 2016;4:569-73. [Crossref] [PubMed]
- Ravi P, Mantia C, Su C, et al. Evaluation of the Safety and Efficacy of Immunotherapy Rechallenge in Patients With Renal Cell Carcinoma. JAMA Oncol 2020;6:1606-10. [Crossref] [PubMed]
- Gul A, Shah NJ, Mantia C, et al. Ipilimumab plus nivolumab (Ipi/Nivo) as salvage therapy in patients with immunotherapy (IO)-refractory metastatic renal cell carcinoma (mRCC). J Clin Oncol 2019;37:669. [Crossref]
- Zhang T, Xie J, Arai S, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget 2016;7:73068-79. [Crossref] [PubMed]
- Fujisaki T, Watanabe S, Ota T, et al. The Prognostic Significance of the Continuous Administration of Anti-PD-1 Antibody via Continuation or Rechallenge After the Occurrence of Immune-Related Adverse Events. Front Oncol 2021;11:704475. [Crossref] [PubMed]
- Fujita K, Uchida N, Kanai O, et al. Retreatment with pembrolizumab in advanced non-small cell lung cancer patients previously treated with nivolumab: emerging reports of 12 cases. Cancer Chemother Pharmacol 2018;81:1105-9. [Crossref] [PubMed]
- Fujita K, Uchida N, Yamamoto Y, et al. Retreatment With Anti-PD-L1 Antibody in Advanced Non-small Cell Lung Cancer Previously Treated With Anti-PD-1 Antibodies. Anticancer Res 2019;39:3917-21. [Crossref] [PubMed]
- Fujita K, Yamamoto Y, Kanai O, et al. Retreatment with anti-PD-1 antibody in non-small cell lung cancer patients previously treated with anti-PD-L1 antibody. Thorac Cancer 2020;11:15-8. [Crossref] [PubMed]
- Furuya N, Nishino M, Wakuda K, et al. Real-world efficacy of atezolizumab in non-small cell lung cancer: A multicenter cohort study focused on performance status and retreatment after failure of anti-PD-1 antibody. Thorac Cancer 2021;12:613-8. [Crossref] [PubMed]
- Giaj Levra M, Cotté FE, Corre R, et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: A national data base analysis. Lung Cancer 2020;140:99-106. [Crossref] [PubMed]
- Gobbini E, Toffart AC, Pérol M, et al. Immune Checkpoint Inhibitors Rechallenge Efficacy in Non-Small-Cell Lung Cancer Patients. Clin Lung Cancer 2020;21:e497-510. [Crossref] [PubMed]
- Katayama Y, Shimamoto T, Yamada T, et al. Retrospective Efficacy Analysis of Immune Checkpoint Inhibitor Rechallenge in Patients with Non-Small Cell Lung Cancer. J Clin Med 2019;9:102. [Crossref] [PubMed]
- Kitagawa S, Hakozaki T, Kitadai R, et al. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: Case series and literature review. Thorac Cancer 2020;11:1927-33. [Crossref] [PubMed]
- Koyauchi T, Inui N, Karayama M, et al. Clinical Outcomes of Anti-programmed Death-1 Antibody–Related Pneumonitis in Patients with Non-Small Cell Lung Cancer. SN Compr Clin Med 2020;2:570-8. [Crossref]
- Mouri A, Kaira K, Yamaguchi O, et al. Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother Pharmacol 2019;84:873-80. [Crossref] [PubMed]
- Niki M, Nakaya A, Kurata T, et al. Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget 2018;9:32298-304. [Crossref] [PubMed]
- Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res 2018;6:1093-9. [Crossref] [PubMed]
- Sheth S, Gao C, Mueller N, et al. Durvalumab activity in previously treated patients who stopped durvalumab without disease progression. J Immunother Cancer 2020;8:e000650. [Crossref] [PubMed]
- Takahama T, Takeda M, Haratani K, et al. Efficacy of Re-Treatment with Immune Checkpoint Inhibitors in Patients with Pretreated Advanced Non-Small Cell Lung Carcinoma. J Thorac Oncol 2018;13:S933. [Crossref]
- Watanabe H, Kubo T, Ninomiya K, et al. The effect and safety of immune checkpoint inhibitor rechallenge in non-small cell lung cancer. Jpn J Clin Oncol 2019;49:762-5. [Crossref] [PubMed]
- Inno A, Roviello G, Ghidini A, et al. Rechallenge of immune checkpoint inhibitors: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2021;165:103434. [Crossref] [PubMed]
- Magee DE, Hird AE, Klaassen Z, et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann Oncol 2020;31:50-60. [Crossref] [PubMed]
- Dupont R, Bérard E, Puisset F, et al. The prognostic impact of immune-related adverse events during anti-PD1 treatment in melanoma and non-small-cell lung cancer: a real-life retrospective study. Oncoimmunology 2019;9:1682383. [Crossref] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Girard N, Jacoulet P, Gainet M, et al. Third-line chemotherapy in advanced non-small cell lung cancer: identifying the candidates for routine practice. J Thorac Oncol 2009;4:1544-9. [Crossref] [PubMed]
- Costantini A, Corny J, Fallet V, et al. Efficacy of next treatment received after nivolumab progression in patients with advanced nonsmall cell lung cancer. ERJ Open Res 2018;4:e00120-2017. [Crossref] [PubMed]
- Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol 2019;5:1310-7. [Crossref] [PubMed]
- Abou Alaiwi S, Xie W, Nassar AH, et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J Immunother Cancer 2020;8:e000144. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. KEYNOTE-024 3-year survival update: pembrolizumab versus platinum-based chemotherapy for advanced NSCLC. J Thorac Oncol 2019;14:S243. [Crossref]
- Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol 2021;39:4073-126. [Crossref] [PubMed]
- Reid PD, Cifu AS, Bass AR. Management of Immunotherapy-Related Toxicities in Patients Treated With Immune Checkpoint Inhibitor Therapy. JAMA 2021;325:482-3. [Crossref] [PubMed]
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119-42. [Crossref] [PubMed]
- Weber JS, Yang JC, Atkins MB, et al. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol 2015;33:2092-9. [Crossref] [PubMed]
- Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016;27:559-74. [Crossref] [PubMed]
- Haratani K, Hayashi H, Chiba Y, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol 2018;4:374-8. [Crossref] [PubMed]
- Bernard-Tessier A, Baldini C, Martin P, et al. Outcomes of long-term responders to anti-programmed death 1 and anti-programmed death ligand 1 when being rechallenged with the same anti-programmed death 1 and anti-programmed death ligand 1 at progression. Eur J Cancer 2018;101:160-4. [Crossref] [PubMed]
- Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol 2020;15:1351-60. [Crossref] [PubMed]
- Garassino MC, Gadgeel S, Esteban E, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:387-97. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]


