
Efficacy and safety of first-line anlotinib-based combinations for advanced non-small cell lung cancer: a three-armed prospective study
Introduction
After years of development, targeted therapy, chemotherapy, and immunotherapy have become the 3 major treatments for advanced lung cancer. Although these treatments have conferred significant survival benefits to patients, in order to further enhance efficacy and prolong survival, combined therapy has become a new treatment strategy.
Antiangiogenic agents targeting the vascular endothelial growth factor (VEGF)-VEGF receptor (VEGFR) pathway include bevacizumab interacting with VEGF (1), play important roles in the combined regimens for patients with advanced lung cancer. In combination with chemotherapy, bevacizumab plus paclitaxel/carboplatin has shown a median overall survival (OS) of 12.3 months compared with 10.3 months for paclitaxel/carboplatin in patients with metastatic non-small cell lung cancer (NSCLC) (2). In addition, multi-target tyrosine kinase inhibitors (TKIs), such as erlotinib can competitively bind to the intracellular tyrosine kinase domain, inhibit its phosphorylation, and block the activation of downstream signaling pathways, thereby inhibiting tumor angiogenesis. The combination of erlotinib and bevacizumab has achieved a longer median progression-free survival (PFS) of 17.9 months than the 13.5 months of erlotinib alone for epidermal growth factor receptor (EGFR)-mutant NSCLC (3), while osimertinib plus bevacizumab achieved a PFS of 15.4 months in patients with metastatic EGFR-mutant lung cancers (4). Additionally, the IMpower150 trial demonstrated enhanced efficacy for atezolizumab and bevacizumab combined with chemotherapy in chemotherapy-naïve patients, making this regimen one of the standard treatment options for advanced non-squamous NSCLC (5). However, current studies of combined therapies for NSCLC have mainly focused on single target monoclonal antibody, antiangiogenic agents. Most of the small-molecule antiangiogenic agents have had negative results, although the combined treatment of nintedanib with docetaxel has achieved positive results as a second-line treatment for adenocarcinoma (6-8). Failure of these multi-target drug combinations is due to high toxicity or poor efficacy.
Fortunately, the China-innovated TKI, anlotinib, has become the first multi-target antiangiogenic agent approved for lung cancer in China due to high efficacy and low side effects and has become a new third-line treatment for patients with advanced NSCLC, as recommended by the 2020 Chinese Society of Clinical Oncology (CSCO) Guideline for NSCLC (9). Compared with bevacizumab and ramucirumab, anlotinib has the advantages of high single-agent effect, effectiveness in patients with squamous cell carcinoma, and convenient application of oral administration. As the only effective monotherapy among antiangiogenic agents as third-line treatment for patients with advanced NSCLC, anlotinib has been shown to prolong OS by 3.3 months compared with placebo [9.6 vs. 6.3 months, hazard ratio (HR) =0.68] (10). Real-world studies demonstrated the feasibility and preliminary effectiveness of anlotinib combined with immunotherapy (11,12) target therapy and chemotherapy (13) in first- or second-line setting. However, the efficacy and safety of anlotinib-based combination in first-line treatment of advanced NSCLC was yet to explored.
Considering the satisfying efficacy and manageable toxicity of anlotinib, we aimed to carry out an exploratory study examining the combinations of EGFR-TKI, chemotherapy, and an immune checkpoint inhibitor (ICI) with anlotinib as the first-line treatment of locally advanced or metastatic NSCLC, to evaluate the efficacy and adverse reactions of the combinations of these main treatments with anlotinib. This study might provide multiple treatment options for advanced NSCLC. We present the following article in accordance with the TREND reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-438/rc).
Methods
Study design and patients
This single-center, exploratory, three-arm trials enrolled patients with NSCLC who were admitted to the Department of Respiratory Medicine at Shanghai Chest Hospital were enrolled between July 2018 and April 2019.
The key inclusion criteria were as follows: (I) 18–75 years of age; (II) histologically diagnosed with unresectable NSCLC of tumor-node-metastasis (TNM) stage IIIb, IIIc, or IV, as defined by the American Joint Committee on Cancer 8th Edition (AJCC8) and with at least 1 measurable lesion according to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) criteria; (III) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (IV) no previous systemic treatments for advanced diseases; (V) sufficient tumor tissue samples for molecular detection to determine the driver gene status. The key exclusion criteria were as follows: (I) known symptomatic brain metastases, spinal cord compression, or carcinomatous meningitis; (II) central type cavernous squamous cell carcinoma, hemorrhagic symptoms, or hemorrhage tendency; (III) hemorrhage tendency (e.g., active gastrointestinal ulcer) treated with anti-coagulants or vitamin K antagonists (including warfarin, heparin, or analogs); (IV) history of arterial/venous thromboembolism before the first dosing, including cerebrovascular accidents (e.g., transient cerebral ischemic attacks), deep venous thrombosis, and pulmonary embolism.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai Chest Hospital (No. KS1840). All participants provided signed informed consent. The trial was registered at clinicaltrials.gov (NCT03628521).
Procedure
In patients with EGFR mutant NSCLC, oral administration of erlotinib (150 mg, QD) was performed alongside oral intake of 10 mg anlotinib (cohort A). Anlotinib was administered for 2 continuous weeks, followed by an interval of 1 week, which was considered 1 treatment cycle (21 days).
In patients with no EGFR/anaplastic lymphoma kinase (ALK)/receptor tyrosine kinase (ROS1) mutation NSCLC, the treatment regimen comprised anlotinib in combination with chemotherapy or ICI [programmed cell death protein 1 (PD-1) inhibitor]. Patients were enrolled in the chemotherapy group first and then in the ICI group.
In cases administered with anlotinib combined with chemotherapy (cohort B), the regimen was as follows: pemetrexed for adenocarcinoma (500 mg/m2) or gemcitabine for squamous carcinoma (1,000 mg/m2) on day (d)1 and d8, combined with carboplatin [area under the curve (AUC) =5] every 3 weeks and anlotinib (oral intake, 12 mg, QD on day 1 to 14 per cycle). A total of 4–6 cycles of chemotherapy, then pemetrexed and anlotinib were continued to maintain treatment until disease progression, appearance of intolerable toxic effects, or withdrawal of informed consent.
In individuals administered with anlotinib combined with ICI (cohort C), the standard dose of sintilimab (200 mg, intravenous injection once every 3 weeks) was combined with the standard dose of anlotinib (oral intake, 12 mg QD day 1–14 every cycle).
The dose of anlotinib or erlotinib would be cut down by 20% when ≥ grade 3 non-hematological adverse events (AEs) or grade 4 hematological AEs occurred and improved to grade 1 in 2 weeks, otherwise withdrawn from the study. Treatments were performed for at least 2 cycles (42 days).
The baseline characteristics, such as demographic characteristics, clinical stage, pathologic diagnosis, brain metastases, EGFR mutations and PD-L1 expression were collected. Before the treatment, the patients’ tumor tissue samples would be collected for programmed death-ligand 1 (PD-L1) expression evaluation and tumor mutation burden (TMB) assessment. PD-L1 expression was measured by 22C3 pharmDx assay (Agilent Technologies, Carpinteria, CA), and PD-L1–positive (PD-L1+) was defined as PD-L1 tumor proportion score greater than or equal to 1%. The TMB was measured by the FoundationOne CDx assay (Foundation Medicine, Cambridge, MA), and TMB-high (TMB-H) was defined as greater than or equal to 10 mutations per Mb.
Outcomes
The primary endpoints were safety and objective response rate (ORR). Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE 4.0) by the National Cancer Institute (14). The ORR was defined as the proportion of patients with complete response (CR) or partial response (PR).
The secondary endpoints included PFS, disease control rate (DCR), and OS. The definition of PFS was the time from the first dose of either drug to investigator-assessed radiological progressive disease (PD) or death from any cause and was censored at the last tumor assessment. The definition of OS was the time from patient enrollment to death from any cause. Disease control rate was defined as the proportion of patients with CR, PR, or stable disease (SD). All patients underwent brain magnetic resonance imaging at baseline, and for those with brain metastases, tumor response as well as the intracranial response be evaluated per Response Evaluation Criteria in Solid Tumors 1.1 concurrently at scheduled tumor assessments. Efficacy was evaluated every 2 cycles using RECIST version 1.1. Safety was assessed throughout the study. All patients would be followed up every 2 months for survival after PD.
Statistical analysis
All efficacy endpoints were assessed in the full analysis set (intent-to-treat principle), which included all patients who met the inclusion and exclusion criteria. Safety was assessed in all participants who received at least 1 dose of study treatment.
Statistical analysis was performed with SAS 9.4 (SAS Institute, Cary, NC, USA). Continuous variables were presented as means ± standard deviations or medians and ranges. Categorical variables were presented as n (%). The ORR and DCR were calculated with corresponding 2-sided 95% confidence intervals (CI) using the Clopper-Pearson method. Both PFS and OS were estimated by the Kaplan-Meier method. Only descriptive statistical analysis was performed.
Results
Baseline patient characteristics
A total of 82 patients were enrolled, including 30, 30, and 22 patients in cohorts A, B, and C, respectively, between July 2018 and April 2019. The baseline characteristics of patients, including age, gender, pathology, and Eastern Cooperative Oncology Group (ECOG) performance status, are shown in Table 1. There were 3 patients in cohort A did not complete the treatment. The data cutoff for this analysis was 31 December 2020. The median follow-up durations were 22.8 (range, 0.1–26.7), 25.7 (range, 2.8–29.4), and 23.4 (range, 4.5–27.2) months in cohorts A, B, and C, respectively.
Table 1
| Characteristics | Anlotinib + erlotinib (cohort A) (n=30) | Anlotinib + pemetrexed/gemcitabine + carboplatin (cohort B) (n=30) | Anlotinib + sintilimab (cohort C) (n=22) |
|---|---|---|---|
| Age (years), median [range] | 56 [41–76] | 64.5 [47–76] | 64.5 [47–74] |
| Gender, n (%) | |||
| Male | 13 (43.3) | 23 (76.7) | 21 (95.5) |
| Female | 17 (56.7) | 7 (23.3) | 1 (4.5) |
| Pathology, n (%) | |||
| Adenocarcinoma | 27 (90.0) | 23 (76.7) | 9 (40.9) |
| Adenosquamous | 3 (10.0) | 0 | 0 |
| Squamous | 0 | 7 (23.3) | 12 (54.5) |
| Not otherwise specified | 0 | 0 | 1 (4.5) |
| Stage, n (%) | |||
| IIIb | 1 (3.3) | 4 (13.3) | 4 (18.2) |
| IIIc | 0 | 0 | 5 (22.7) |
| IV | 29 (96.7) | 26 (86.7) | 13 (59.1) |
| Brain metastases, n (%) | |||
| Yes | 9 (30.0) | 0 | 4 (18.2) |
| No | 21 (70.0) | 30 (100.0) | 18 (81.8) |
| Smoking, n (%) | |||
| Yes | 8 (26.7) | 18 (60.0) | 14 (63.6) |
| No | 22 (73.3) | 12 (40.0) | 8 (36.4) |
| ECOG PS, n (%) | |||
| 0 | 3 (10.0) | 0 | 1 (4.5) |
| 1 | 27 (90.0) | 30 (100.0) | 21 (95.5) |
| EGFR mutation, n (%) | |||
| 19deletion | 18 (60.0) | 0 | 0 |
| L858R | 11 (36.7) | 0 | 0 |
| 18G719, 21L861Q | 1 (3.3) | 0 | 0 |
| TP53 co-mutation, n (%) | |||
| Yes | 17 (56.7) | – | – |
| No | 13 (43.3) | – | – |
| PD-L1 TPS, n (%) | |||
| <1% | – | – | 8 (36.4) |
| 1–49% | – | – | 5 (22.7) |
| ≥50% | – | – | 8 (36.4) |
| NE | – | – | 1 (4.5) |
| TMB status, n (%) | |||
| ≥10 Muts/Mb | – | – | 7 (31.8) |
| <10 Muts/Mb | – | – | 11 (50.0) |
| NE | – | – | 4 (18.2) |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; EGFR, epidermal growth factor receptor; PD-L1, programmed death-ligand 1; TMB, tumor mutation burden; TPS, tumor proportion score; NE, not evaluable.
AEs
At the cutoff date, participants had different degrees of AEs, both in cohorts A and B (Tables 2,3).
Table 2
| Treatment emergent AEs | Anlotinib + erlotinib (cohort A) (n=30) | Anlotinib + pemetrexed/gemcitabine + carboplatin (cohort B) (n=30) |
|---|---|---|
| AEs, n (%) | 30 (100.0) | 30 (100.0) |
| Treatment-related AEs, n (%) | 30 (100.0) | 30 (100.0) |
| Severe AEs (grade ≥3), n (%) | 22 (73.3) | 18 (60.0) |
| Treatment-related severe AEs (grade ≥3), n (%) | 22 (73.3) | 18 (60.0) |
| Serious AEs, n (%) | 4 (13.3) | 3 (10.0) |
| Treatment-related serious AEs, n (%) | 4 (13.3) | 3 (10.0) |
| AEs leading to dose interruption, n (%) | 9 (30.0) | 6 (20.0) |
| AEs leading to dose adjustment, n (%) | 12 (40.0) | 5 (16.7) |
| AEs leading to treatment discontinuation, n (%) | 3 (10.0) | 3 (10.0) |
| AEs leading to death, n (%) | 0 (0.0) | 0 (0.0) |
AEs, adverse events.
Table 3
| AEs | Anlotinib + erlotinib (cohort A) (n=30), n (%) | Anlotinib + pemetrexed/gemcitabine + carboplatin (cohort B) (n=30), n (%) | |||
|---|---|---|---|---|---|
| TRAEs of any grade with occurrence ≥20% | TRAEs of ≥ grade 3 | TRAEs of any grade with occurrence ≥20% | TRAEs of ≥ grade 3 | ||
| Leucopenia | – | – | 30 (100.0) | 5 (16.7) | |
| Decreased platelet count | – | – | 27 (90.0) | 9 (30.0) | |
| Hand-foot syndrome | – | – | 27 (90.0) | 3 (10.0) | |
| Hypertriglyceridemia | 10 (33.3) | 1 (3.3) | 20 (66.7) | 3 (10.0) | |
| Pharyngalgia | – | – | 19 (63.3) | – | |
| Diarrhea | 12 (40.0) | 2 (6.7) | 17 (56.7) | – | |
| Hypertension | 20 (66.7) | 2 (6.7) | 15 (50.0) | – | |
| Decreased appetite | 12 (40.0) | – | 14 (46.7) | – | |
| Hypercholesteremia | 9 (30.0) | – | 13 (43.3) | – | |
| Anemia | – | – | 12 (40.0) | – | |
| Oral mucositis | 19 (63.3) | 3 (10.0) | 11 (36.7) | 2 (6.7) | |
| Hyperuricemia | – | – | 10 (33.3) | 1 (3.3) | |
| Rash | 30 (100.0) | 5 (16.7) | – | – | |
| Proteinuria | 11 (36.7) | 2 (6.7) | – | – | |
| TSH increase | 11 (36.7) | 1 (3.3) | 7 (23.3) | – | |
| Thrombus | – | – | – | 2 (6.7) | |
| Hematuria | – | 1 (3.3) | 8 (26.7) | – | |
| Lipase elevation | – | 1 (3.3) | – | – | |
| ALT increased | 7 (23.3) | 1 (3.3) | 6 (20.0) | – | |
| AST increased | 7 (23.3) | 1 (3.3) | – | – | |
| GGT increased | 8 (26.7) | – | – | – | |
| Hemobilirubin increase | 9 (30.0) | – | – | – | |
| Fatigue | – | – | 7 (23.3) | – | |
| Nausea | 7 (23.3) | – | – | – | |
AE, adverse events; TRAE, treatment-related adverse event; TSH, thyroid stimulating hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase.
In cohort A, 12 (40.0%) participants had dosage adjustment due to AEs (11 participants reduced the dosage of anlotinib and 1 participant reduced the dosage of both anlotinib and erlotinib), 9 (30%) participants had dose interruption of anlotinib due to AEs, and 3 (10.0%) terminated the treatment due to AEs (Table 2). Treatment-related AEs (TRAEs) of grade-3 or higher occurred in 77.3% (22/30) of cohort A. The most common TRAEs of ≥ grade-3 were rash (16.7%), oral mucositis (10.0%), diarrhea (6.7%), hypertension (6.7%), proteinuria (6.7%), alanine aminotransferase (ALT) increase (6.7%), and aspartate aminotransferase (AST) increase (6.7%), and grade-4 hypertension was observed in 1 participant with a history of hypertension (Table 3). No patient died due to AEs in cohort A.
In cohort B, 5 (16.7%) participants had dosage adjustment (2 patients reduced the dosage of anlotinib and 3 patients reduced the dosage of chemotherapy), 6 (20.0%) participants had dose interruption (3 patients with chemotherapy delayed and 3 patients with both anlotinib and chemotherapy delayed) due to AEs, and 3 participants (10.0%) terminated their treatment due to AEs (Table 2). In cohort B, TRAEs of grade-3 or worse occurred in 60.0% (18/30) of participants. The most common ≥ grade-3 TRAEs were decreased platelet count (30.0%), leucopenia (16.7%), hand-foot syndrome (10.0%), hypertriglyceridemia (10.0%), oral mucositis (6.7%), and thrombus (6.7%) (Table 3). In squamous cell carcinoma cases administered anlotinib combined with gemcitabine and carboplatin, 3 participants (10.0%) presented with grade-4 decreased platelet count (Table 3). No patient died due to AEs in cohort B.
The safety data of cohort C have been published previously (15).
Outcomes
In cohort A, 26 (86.7%) and 1 (3.3%) participant showed PR and SD, respectively (Figure 1) and 3 patients were not evaluable. The ORR was 86.7% (95% CI: 69.3–96.2%) in cohort A and the DCR was 90.0% (95% CI: 73.5–97.9%) (Table S1). At the cutoff date, 16 participants had disease progression. The median PFS (mPFS) was 21.6 months (95% CI: 15.6–24.9 months) (Figure 2A). The median OS (mOS) was not reached, and only 3 participants died. The 12- and 24-month OS rates were 100.0% (95% CI: 100.0–100.0%) and 87.1% (95% CI: 63.8–95.8%), respectively (Figure 2B). Among the 9 (30.0%) participants with brain metastasis at baseline in cohort A, no patient received radiation. Among 7 patients with measurable brain lesions, three achieved intracranial CR and 2 had intracranial PR; 1 showed SD. In the two patients with unmeasurable brain lesions, the lesions were obviously reduced. The intracranial ORR (iORR) was 55.6%, and intracranial DCR (iDCR) was 88.9%. At the last follow-up, only 1 participant had developed new brain lesions (Table S2).
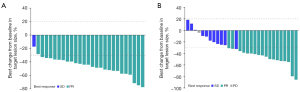
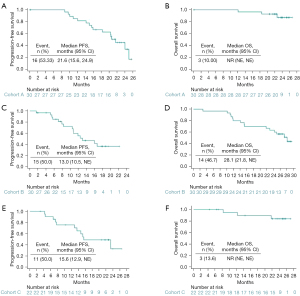
The ORR was 60.0% (95% CI: 40.6–77.3%) in cohort B. Totals of 18 (60.0%) and 11 (36.7%) participants showed PR and SD, respectively (Figure 1). Only 1 (3.3%) participant showed PD due to increased amounts of liver metastases. The DCR was 96.7% (95% CI: 82.8–99.9%) (Table S1). At the cutoff date, 15 participants had disease progression. The mPFS was 13.0 months (95% CI: 10.5 months–NE) (Figure 2C). In this cohort, 14 participants died, and the mOS was 28.1 months (95% CI: 21.8 months–NE) (Figure 2D).
The ORR and DCR data in cohort C had been published previously (15). At the cutoff date, 11 participants had PD. The updated mPFS was 15.6 months (95% CI: 12.9 months–NE) (Figure 2E). Only 3 participants died, and the updated 12- and 24-month OS rates were 94.7% (95% CI: 68.1–99.2%) and 83.9% (95% CI: 57.9–94.5%), respectively (Figure 2F).
Subgroup analysis
In subgroup analysis of different mutation types in cohort A, there was no significant difference in mPFS between patients with EGFR exon 19 deletion (EGFR 19del) and 21L858R (24.0 vs. 20.4 months; HR =0.62, 95% CI: 0.23–1.67; P=0.3399) (Figure 3A). The mPFS in patients without TP53 co-mutation was 21.0 months compared with 24.0 months in those with TP53 co-mutation (HR =1.08; 95% CI: 0.39–3.00; P=0.8777) (Figure 3B). Participants with brain metastasis achieved an mPFS of 24.0 months, while those without brain metastasis had an mPFS of 20.4 months (HR =0.66; 95% CI: 0.21–2.09; P=0.4787) (Figure 3C).
Discussion
For the first time, this exploratory, 3-armed study evaluated the safety and efficacy of the multi-target antiangiogenic TKI anlotinib, combined with present standard strategies as a first-line treatment of advanced NSCLC. The results indicated that the 3 regimens of anlotinib combined with EGFR-TKI, chemotherapy, and PD-1 inhibitor all showed good safety and encouraging efficacy.
Multiple prospective studies confirmed that VEGF/VEGFR inhibitors combined with EGFR-TKI could prolong PFS. The NEJ026 trial showed that bevacizumab plus erlotinib improved efficacy compared with erlotinib alone in Japanese patients with an ORR of 72% and a PFS of 16.9 months (16). The CTONG1509 study showed that bevacizumab plus erlotinib had an ORR of 86.3% and a PFS of 18.0 months in Chinese patients (17). The RELAY study showed ramucirumab plus erlotinib had an ORR of 76% (18) and a PFS of 19.4 months (19). In these previous studies, 72–88% of the patients treated with combined therapies had grade-3 or worse AEs, while 73.3% of cohort A had grade-3 or worse TRAEs in this study. In cohort A in our study, the most common TRAEs were rash, oral mucositis, diarrhea, hypertension, proteinuria, ALT increase, and AST increase, and grade-4 hypertension was observed in one patient, similar to the safety results in erlotinib (20) or anlotinib alone (10). The observed ORR was as high as 86.7% and the mPFS was 21.6 months. Unprecedented improvements in ORR and PFS may be due to the wider target range of anlotinib compared with bevacizumab or ramucirumab. However, this was a small sample study, and 40.0% of the patients had their dosage adjusted due to AEs. This suggests that longer follow-up and more samples are needed before concluding on efficacy. The most appropriate dose of the combination of these 2 TKIs remain to be determined.
Additionally, 9 patients with brain metastasis were enrolled in cohort A, including 3 with single brain lesions and 6 with multiple brain lesions. Among these patients, no patient received radiation and the intracranial treatment response of systemic therapy was promising. To date, only 1 participant has developed new brain lesions. Given the small sample size of the subgroups in this study, further studies were warranted to explore the intracranial response of anlotinib and erlotinib.
Previous studies have shown TKI treatment is less effective in patients with EGFR L858R mutation than in those with EGFR 19del (21). Besides, the effect of TKI treatment in patients with TP53 co-mutation is also less potent than in those without such co-mutation (22). In cohort A of this study, patients with EGFR 21 L858R accounted for 36.7%, and those with TP53 co-mutation accounted for 56.7%. The results could suggest that there was no significant difference in mPFS between patients with EGFR 19del and EGFR 21 L858R, as well as between patients with TP53 co-mutation and those without, suggesting that this combined regimen might benefit patients with both sensitizing EGFR mutations, whether TP53 is co-mutated or not. The subgroup analyses must be taken with caution due to the small sample size in this study. A phase II extended study is being performed to further clarify the relationship between these factors and the efficacy of this combination treatment.
The most common grade-3 or worse TRAEs were decreased platelet count, leucopenia, hand-foot syndrome, hypertriglyceridemia, oral mucositis, and thrombus. A total of 3 patients with grade-4 decreased platelet count all had squamous cell carcinoma, and none of them had bleeding symptoms. However, their side effects were overlapped because anlotinib, gemcitabine, and carboplatin all have partial platelet lowering effects. Thus, we only selected patients with non-squamous NSCLC for the subsequent ongoing multicenter phase III study assessing the combination of anlotinib with pemetrexed and carboplatin. In this cohort, ORR was 60.0%, and mPFS and mOS were 13.0 months and 28.1 months, respectively. While, in the BEYOND study, the first-line treatment of bevacizumab plus platinum doublet chemotherapy achieved an ORR of 54% and an mPFS of 9.2 months in the Chinese patients with advanced NSCLC (23). Therefore, a multicenter, randomized, double-blinded, placebo-controlled phase III clinical trial (NCT04439890) of 300 patients was conducted to verify these results. The indications of anlotinib include squamous cell carcinoma. Although we found grade-4 decreased platelet count in patients with squamous cell carcinoma who were administered anlotinib combined with gemcitabine and carboplatin in this exploratory study, it is necessary to perform further research to explore the optimal dose and combination of anlotinib as a first-line treatment of squamous cell carcinoma. In cohort C, the safety of anlotinib plus PD-1 inhibitors was tolerable, with low incidences of TRAEs of grade-3 or worse and serious AEs (15). The updated mPFS was 15.6 months, which was longer than the similar study reported previously (5). Further multicenter randomized controlled trials are also in progress. Of note, the LEAP-007 trial of pembrolizumab with or without lenvatinib was terminated based on the prespecified futility criteria (24).
Although this study firstly explored the safety and efficacy of the multi-target antiangiogenic agent combined with current standard therapeutics as first-line treatment of advanced NSCLC, due to the small sample size and single-center design, limitations were unavoidable. With the primary endpoints of safety and ORR, mOS was immature, limiting the evaluation of long-term survival benefits. Osimertinib is now the stand of care, but the use of osimertinib as a first-line treatment of EGFR-mutated advanced NSCLC was approved by the National Medical Products Administration in China in August 2019, which was after the trial was designed and enrollment started. Similarly, the 2018 NCCN guideline-recommended pembrolizumab as a subsequent option of progression after first-line systemic therapy. Hence, with the rapid progress of the treatment paradigm of advanced NSCLC, the generalization of findings of this study might be limited. We hope to provide a reference for the next step in larger sample future studies.
Conclusions
Anlotinib in combination with EGFR-TKI, chemotherapy, or ICI could achieve an acceptable safety profile in the first-line treatment of advanced NSCLC, with satisfactory treatment effects. Combination treatments, including anlotinib might provide multiple choices for patients with advanced NSCLC, which deserves further investigation in multicenter randomized controlled studies with a larger sample size. Several randomized controlled trials are ongoing, including NCT04124731 and NCT04439890.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This work was supported by the Western Medicine Guiding Project of Shanghai Science and Technology Commission (No. 18411968500), the Multi-center Clinical Research Project of School of Medicine, Shanghai Jiao Tong University (No. DLY201816), the Shanghai Chest Hospital Cooperative Innovation Project (No. YJXT20190102), and the key project of Science and Technology Commission of Shanghai Municipality (No. 21Y11913500).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-438/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-438/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-438/coif). JWN reports grants and personal fees from Takeda during the conduct of the study; grants, personal fees, and non-financial support from Genentech/Roche and Exelixis; personal fees from AstraZeneca, Jounce Therapeutics, Eli Lilly and Company, Calithera Biosciences, Amgen, Iovance Biotherapeutics, Blueprint Pharmaceuticals, Regeneron Pharmaceuticals, Natera, Surface Oncology, D2G Oncology, Sanofi Genzyme, and Turning Point Therapeutics; grants and non-financial support from Merck, Novartis, Boehringer Ingelheim, Nektar Therapeutics, Adaptimmune, GSK, Janssen, and AbbVie, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai Chest Hospital (No. KS1840). All participants provided signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004;3:391-400. [Crossref] [PubMed]
- Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol 2010;5:1416-23. [Crossref] [PubMed]
- Stinchcombe TE, Jänne PA, Wang X, et al. Effect of Erlotinib Plus Bevacizumab vs Erlotinib Alone on Progression-Free Survival in Patients With Advanced EGFR-Mutant Non-Small Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1448-55. [Crossref] [PubMed]
- Soo RA, Han JY, Dafni U, et al. A randomised phase II study of osimertinib and bevacizumab versus osimertinib alone as second-line targeted treatment in advanced NSCLC with confirmed EGFR and acquired T790M mutations: the European Thoracic Oncology Platform (ETOP 10-16) BOOSTER trial. Ann Oncol 2022;33:181-92. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Hanna NH, Kaiser R, Sullivan RN, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-Lung 2): A randomized, double-blind, phase III trial. Lung Cancer 2016;102:65-73. [Crossref] [PubMed]
- Buddharaju LNR, Ganti AK. Immunotherapy in lung cancer: the chemotherapy conundrum. Chin Clin Oncol 2020;9:59. [Crossref] [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [Crossref] [PubMed]
- Chinese Society of Clinical Oncology. Guidelines of Chinese Society of Clinical Oncology (CSCO) Non-small Cell Lung Cancer. Beijing: People's Medical Publishing House Co., LTD; 2020.
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Xiong Q, Qin B, Xin L, et al. Real-World Efficacy and Safety of Anlotinib With and Without Immunotherapy in Advanced Non-Small Cell Lung Cancer. Front Oncol 2021;11:659380. [Crossref] [PubMed]
- Zhang X, Zeng L, Li Y, et al. Anlotinib combined with PD-1 blockade for the treatment of lung cancer: a real-world retrospective study in China. Cancer Immunol Immunother 2021;70:2517-28. [Crossref] [PubMed]
- Li L, Zhang H, Xie Y, et al. The Efficacy and Safety of Anlotinib Alone and in Combination with Other Drugs in Advanced Lung Cancer: A Retrospective Cohort Study. Comput Math Methods Med 2022;2022:1475871. [Crossref] [PubMed]
- Institute NC. Common Terminology Criteria for Adverse Events. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
- Chu T, Zhong R, Zhong H, et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J Thorac Oncol 2021;16:643-52. [Crossref] [PubMed]
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019;20:625-35. [Crossref] [PubMed]
- Zhou Q, Wu YL, Cheng Y, et al. 1480O - CTONG 1509: Phase III study of bevacizumab with or without erlotinib in untreated Chinese patients with advanced EGFR-mutated NSCLC. Ann Oncol 2019;30:v603. [Crossref]
- Nishio M, Seto T, Reck M, et al. Ramucirumab or placebo plus erlotinib in EGFR-mutated, metastatic non-small-cell lung cancer: East Asian subset of RELAY. Cancer Sci 2020;111:4510-25. [Crossref] [PubMed]
- Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:1655-69. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Zhou J, Ben S. Comparison of therapeutic effects of EGFR-tyrosine kinase inhibitors on 19Del and L858R mutations in advanced lung adenocarcinoma and effect on cellular immune function. Thorac Cancer 2018;9:228-33. [Crossref] [PubMed]
- Labbé C, Cabanero M, Korpanty GJ, et al. Prognostic and predictive effects of TP53 co-mutation in patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung Cancer 2017;111:23-9. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2197-204. [Crossref] [PubMed]
- Csoszi T, Yang JC, Luft A, et al. 120O - Pembrolizumab (Pembro) With or Without Lenvatinib (Lenva) in First-Line Metastatic NSCLC With PD-L1 TPS ≥1% (LEAP-007): A Phase 3, Randomized, Double-Blind Study. Ann Oncol 2021;32:S1428-57.



