
Locally advanced undifferentiated small round cell sarcoma of the lung with novel SDCCAG8-AKT3 fusion and type II tumor immunity in the microenvironment: a rare case report
Introduction
In 2013, the fourth edition of the World Health Organization (WHO) Classification of Bone and Soft Tissue Tumors firstly introduced the classification of undifferentiated small round cell sarcomas (USRCS). Surgery is the standard treatment of all patients with an adult-type, localized soft tissue sarcoma (STS), including USRCS (1). However, when managing patients with advanced, unresectable STS, the decision-making is complex, depending on the diverse presentations and histologies, and should always be multidisciplinary, chemotherapy, radiotherapy, surgery (post treatment or for symptoms control) and best supportive care might be considered, but immunotherapy isn’t routinely involved yet (2) due to limited evidences.
Despite a shared round cell morphology, and occasional overlap in clinical presentation, additional transcriptional, and epigenetic investigations have clearly established, for example, based on the spread of next-generation sequencing (NGS) technologies, allowed the classification of capicua transcriptional repressor (CIC)-rearranged and Bcl6 corepressor (BCOR)-rearranged sarcomas, as discrete pathologic entities, separate from classic Ewing family tumors (EFTs) (3,4). Importantly, in addition to the morphological and molecular genetic diversity, different gene alternation sarcomas exhibit significant clinical differences. For instance, CIC rearranged sarcomas are more aggressive than Ewing sarcoma (ES) and have a lower response rate to ES chemotherapy regimens (5), while BCOR rearranged sarcoma is more indolent, but is more sensitive to ES chemotherapy (6,7). Nevertheless, a subset of USRCS have not been yet classified, the value of gene fusion alternations in morphologically and immunohistochemically undefined USRCS should not be underestimated.
Although the lung is one of the most common metastatic organs for STS, primary lung USRCS is extremely uncommon. For extraosseous primary tumors, the proportion of primary lung USRCS is unclear and only limited cases reported (8,9). For this rare occurrence of USRCS, the relationship between gene phenotype, pathological phenotype and clinical phenotype, and whether both the pathological characteristics of sarcoma and lung cancer should be taken into consideration when making treatment decision, all deserves further exploring. Herein, we report the case of a young male diagnosed with unresectable lung USRCS. Tumor specimens from this patient detected a specific immune-microenvironment phenotype with a 100% programmed death ligand 1 (PD-L1) expression and type II tumor immunity in the microenvironment (type II TIME). We also detected an SDCCAG8-AKT3 (S19:A2) gene fusion that has never been reported in USRCS. The treatment outcome of this patient suggests that chemotherapy combined with immunotherapy and sequential radiotherapy may provide therapeutic benefits for these patients. We present the following case in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-572/rc).
Case presentation
On 28 April 2021, a 31-year-old Chinese male was admitted to the West China Hospital, Sichuan University for cough and dyspnea, accompanied by fatigue and intermittent bloody sputum for 2 months, not fever, chest pain, nausea and vomiting, etc. He did not receive any medications or physiotherapy before admitted to our hospital. He had no history of hypertension, diabetes, exposure to poisons or chemicals, or regular medication, exposure to infectious disease, and no significant family history, no history of smoking and alcohol abuse. A physical examination revealed reduced respiratory sounds in the left lower lung which equated to an Eastern Cooperative Oncology Group (ECOG) score of 1 point.
Chest enhanced computed tomography (CT) showed a huge, irregular mass (11.5 cm × 10.2 cm) in the left lung, which invaded the left main bronchus and pericardium, and fused with left hilar lymph node. Besides, multiple enlarged mediastinal lymph nodes (3.4 cm × 2.9 cm, max) and pleural effusion were revealed in CT scans (Figure 1A; Table 1). The positron-emission tomography/CT (PET/CT) images are shown Figure 1B and the max standard uptake value (SUV) was 26.45. No distant metastasis was detected by PET/CT and head enhanced magnetic resonance imaging (MRI).
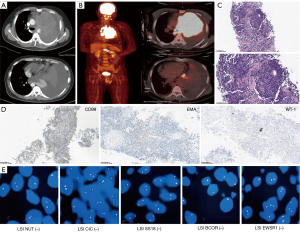
Table 1
| Dates | Initial and follow-up visits | Diagnostic testing (date) | Diagnosis and interventions |
|---|---|---|---|
| Apr. 2021 | The patient complained of cough and dyspnea | Chest enhanced CT: a large mass in the left lung, pleural effusion (30/04/2021) | Pulmonary mass: malignancy? |
| Head enhanced MRI: no metastases (06/05/2021) | |||
| May 2021 | In-patient visit | Exfoliative cell examination of sputum: no malignant tumor cells were detected (13/05/2021) | Percutaneous lung biopsy |
| Jun. 2021 | Out-patient visit | Percutaneous lung biopsy: based on IHC, malignant tumor was considered, the differential diagnosis includes sarcoma and poorly differentiated carcinoma, FISH or NGS for gene fusion was suggested (20/05/2021) | Diagnosed as USRCS |
| NGS-DNA: PMS2 mutation, CD274 amplification, TMB-H, MSI-H (10/06/2021) | |||
| NGS-RNA: SDCCAG8-AKT3 (S19:A2) gene fusion (13/06/2021) | |||
| Percutaneous lung biopsy (supplement): FISH analysis showed no Ewing-specific rearrangement of EWSR1 (24/06/2021) | |||
| Jun. – Jul. 2021 | In-patient visit, the patient complained of progressive cough and dyspnea | Chest enhanced CT: pulmonary lesion similar as described above (22/06/2021) | (I) USRCS (cT4N1M0) was diagnosed. (II) The patient received VAC chemotherapy + pembrolizumab 200 mg, q3w, for 2 cycles |
| PET/CT: malignant lesions in the left lung with metastasis of mediastinal lymph nodes. No distant metastases (25/06/2021) | |||
| Thoracentesis: no malignant tumor cells (25/06/2021; 22/07/2021; 23/07/2021) | |||
| Aug.–Nov. 2021 | In-patient visit, the patient’s chief complains relived a lot | Chest enhanced CT: the target lesion of the left lung reduced, pleural effusion controlled well (11/08/2021) | VAC chemotherapy + pembrolizumab (regimens and dosed unchanged), for 4 cycles |
| Response evaluation: PR | |||
| TIME analysis: a type II-TIME type was demonstrated (26/09/2021) | |||
| Chest enhanced CT: the target lesion of the left lung was reduced, PR maintained (01/11/2021) | |||
| Dec. 2021 | The patient’s chief complains relived a lot | None | Radiotherapy: 66 Gy/33 f, IMRT |
| Feb. 2022 | In-patient visit without clinical symptoms | Chest enhanced CT:PR maintained (24/02/2022) | VAC chemotherapy combined with pembrolizumab, for 2 cycles |
| Apr. 2022 | Out-patient visit without clinical symptoms | Chest enhanced CT:PR maintained (14/04/2022) | The patient had returned to his resident city and continuing immunotherapy |
| Aug. 2022 | follow-up by telephone | NONE (08/08/2022) | There is no progression of the patient’s target lesion and he is in good condition |
CT, computed tomography; MRI, magnetic resonance imaging; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; NGS, next generation sequencing; USRCS, undifferentiated small round cell sarcoma; TMB, tumor mutational burden; MSI-H, high microsatellite instability; VAC, vincristine sulfate, dactinomycin, and cyclophosphamide; PET/CT, positron-emission tomography/CT; PD-1, programmed cell death protein 1; PR, partial response; TIME, tumor immunity in the microenvironment; IMRT, intensity-modulated radiation therapy; EWSR1, EWS RNA binding protein 1.
Biopsy was obtained by percutaneous lung biopsy. The morphological study revealed a small round cell tumor (Figure 1C). Immunohistochemical (IHC) staining showed that the tumor cells were diffusely positive for CD99, EMA, and WT-1 (Figure 1D), and negative for CK7, CK5/6, CK, TTF-1, P40, CD56, Syn, CgA, TLE-1, CA, CD30, SALL4, Des, myoD1, S-100, SATB2, and CD34; 90% of tumor cells displayed Ki67 positive-staining. Fluorescence in situ hybridization (FISH) analysis showed no Ewing-specific rearrangement of EWSR1 (Figure 1E). The patient underwent thoracentesis, and no malignant tumor cell was detected in the pleural effusion. For more diagnostic information, tumor sample was further analyzed by DNA and RNA sequencing. The DNA sequencing found PMS2 mutation [c.353+1G>A] (+, 55.71%), CD274 amplification (CN =9.2), high tumor mutational burden (TMB-H; 91.72 Mut/Mb), and high microsatellite instability (MSI-H). More importantly, RNA sequencing allowed for the detection of SDCCAG8-AKT3 (S19:A2) gene fusion (Table 1). Combined with the histological morphology, IHC and gene results, it took nearly 2 months to diagnose the patient of USRCS, and the final stage was cT4N1M0.
Subsequently, the patient received a standard-intensity vincristine sulfate, dactinomycin, and cyclophosphamide (VAC) chemotherapy combined with anti-programmed cell death protein 1 (PD-1) immunotherapy pembrolizumab 200 mg d1 + vincristine 2 mg d1 + doxorubicin 75 mg/m2 d1 + cyclophosphamide 1,200 mg/m2 d1 ivgtt q3w, mesna 400 mg was administrated at 0, 4, and 8 hours after cyclophosphamide infusion to prevent urinary tract toxicity, prophylactic managements of chemotherapy-related myelosuppression was conducted after chemotherapy as well. After two cycles of treatment, the tumor had markedly reduced, the patient achieved a partial response (PR) based on response evaluation criteria in solid tumors (RECIST) 1.1. Then, he received radical radiotherapy [66 Gy/33 f, intensity-modulated radiation therapy (IMRT)] after completion of the sixth cycle of treatment, and maintained a PR at the latest review (Figure 2A; Table 1). In the last two CT scans, the mediastinal solid component seems to increase, which was consistent with the range of radiation field. We considered the change was due to radiotherapy rather than tumor progression.
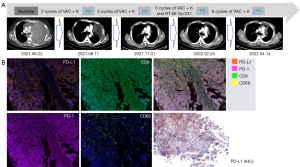
A multiple immunofluorescence (mIF) staining of immune markers of the patient’s tumor sample was conducted to explore the mechanism of his remarkable efficacy, which revealed a type II TIME with PD-L1 (+, 100%), tumor infiltrating lymphocytes (TILs; +, 0.02%), CD68+ macrophages (+, 7.21%), CD68+PD-L1+ macrophages (+, 3.68%), PD-1(−), and CD8+PD-1+ T cells(−) (Figure 2B; Table 1), indicating a high response rate to the anti-PD-1 antibody. The patient is still under immunotherapy in his resident city and no longer experiencing dyspnea, fatigue, cough, bloody sputum, he has returned to normal life and work. During his treatment, blood routine examination, blood biochemistry examination, urine and stool analysis, endocrine function analysis, electrocardiogram (ECG) and enzymatic examination of myocardium were routinely conducted, other than loss of appetite (grade 1, CTCAE5.0) and hair loss after chemotherapy, there were no other adverse events. The duration of response for this case has lasted almost 14 months to the latest follow-up by phone message.
All procedures performed in this study were in accordance with the ethical standards of West China Hospital, Sichuan University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The USRCS, also termed “Ewing-like sarcoma”, is a highly aggressive malignant tumor without Ewing-specific EWSR1 gene rearrangement, which mainly occurs in the trunk and extremities. Its diagnosis and treatment are challenging, with a poor prognosis (4). A primary USRCS in the thoracic visceral organs has rarely been reported since the first case was published in 1991 (8). For unresectable and metastatic STS, chemotherapy including doxorubicin and cyclophosphamide has recommended for decades, although with limited efficacy (1). Some clinical trials of small sample size on advanced sarcoma have indicated that anlotinib (10,11) or immunotherapy (12,13) are potential treatment options in chemotherapy-resistant patients and the objective response rate (ORR) ranges from 13% to 40%. However, a standard treatment for USRCS is still unavailable. A multi-disciplinary discussion including surgeons, medical oncologist, pulmonologist, radiologist, pathologist and radiation oncologists is currently recommended.
To our knowledge, most frequently reported gene fusions in ES-like tumors are CIC-rearrangements and BCOR alternations, however, none of them were detected in our case. Herein, we have firstly reported a PMS2 mutation and a new SDCCAG8-AKT3 (S19:A2) gene fusion in USRCS. The PMS2 gene is located in chromosome 7p22, and PMS2 abnormalities can result in dMMR and MSI-H in endometrial, ovarian, colorectal, and lung cancers (14). Mutation of PMS2 might have contributed to the TMB-H and MSI-H genotype in this young male. An AKT3 fusion is occasionally reported in breast cancer and aggressive angiosarcomas (15,16). As a key molecule in the PI3K/AKT/mTOR signaling pathway, AKT gene activation by AKT fusion is associated with tumorigenesis and drug resistance (15). To date, there is insufficient evidence for the role of PMS2 mutation and SDCCAG8-AKT3 (S19:A2) fusion in the pathogenesis and prognosis of USRCS; more cases and studies are required.
Even though immunotherapy has dramatically improved the prognosis of multiple cancer subtypes, such as melanoma and non-small cell lung cancer (NSCLC), only a minority of patients with STS respond to immune checkpoint inhibitors (ICIs) (17). The tumor microenvironment (TME) has been identified to play an important role in immunotherapy response and acquired resistance (18). In this case, the patient’s TIME may have played an important role in his treatment response. The expression of PD-L1 and TMB in sarcoma is distinctively varied in previously reported cases, and the clinical value in sarcoma, especially in USRCS, is still unclear and underestimated. A published case (19) reported that a patient with undifferentiated sarcoma of the maxillary sinus with PD-L1 >25% achieved complete response (CR) after radiotherapy combined with immunotherapy. Most reports have not detected PD-L1 and TMB in USRCS cases. In our case, the mIF results demonstrated that the patient harbored a type II TIME which was firstly defined by Kim et al. (20) for tumors with both B7-H1 and TILs in the TME, together with CD274 amplification, TMB-H, and MSI-H, all indicating the patient is a beneficial candidate for immunotherapy. Thus, genomic, transcriptomic, and proteomic analyses should be taken into consideration when it comes to diagnosis and treatment of USRCS. The limitation of this study is the short duration of follow up, resulting limited data on the long-term prognosis, we will continue to do the follow-up and hope that he could have a longer survival and maintain a good quality of life.
Conclusions
In conclusion, our knowledge of USRCS is still limited, especially in molecular diagnosis and treatment. Our study showed a rare case of advanced USRCS which primarily occurred in the lung with PMS2 gene mutation and harboring a SDCCAG8-AKT3 (S19:A2) fusion that has not been previously reported, gene sequencing plays an important role in the diagnosis of this case. And we indicated that a comprehensive treatment including the combination of systemic chemotherapy and anti-PD-1 immunotherapy and sequential radiotherapy with promising efficacy and safety which can be considered for similar cases. Prophylactic managements of chemotherapy-related myelosuppression and urotoxicity should be administrated along with chemotherapy, health education including advices on diet, exercise, and on preservation of fertility, and psychological support, should also be considered during treatment. Moreover, TIME analysis is recommended for more prognostic information in addition to routine pathologic examinations in diagnosis and treatment of USRCS.
Questions for further discussion
How to evaluate the value of second-generation sequencing, especially NGS-RNA, in the diagnosis and differential diagnosis of USRCS?
Expert opinion 1: Dr. Massimiliano Bassi
The importance of molecular genetics in soft tissue tumor is unquestionable, especially in USRCS where different entities are defined by specific genetic alterations. Thus, a molecular diagnostic approach based on NGS technology should be preferred if available in case of USRCR.
Expert opinion 2: Dr. Lukas Käsmann
Current studies found up to 30% of all patients with pediatric sarcoma had at least one potentially actionable alteration (21). As a result, NGS, especially NGS-RNA, becomes more important in order to contribute to individualized treatment approaches.
As effective prognostic biomarkers for pembrolizumab in NSCLC, tumor mutational burden and PD-L1 expression have failed to identify good responders in STS. Is tumor immunity in the microenvironment (TIME) a promising prognostic biomarker in STS? Given the complex composition of TIME, such as TILs, tumor-associated macrophages (TAMs), T-regulatory cell, inflammatory factors etc., which path is the most noteworthy?
Expert opinion 1: Dr. Massimiliano Bassi
Surely, PD-L1 expression have not the same prognostic value in STS than in other tumors but I think that it could be still convenient to predict the possible effect of pembrolizumab. TIME could be a promising prognostic biomarker that could replace in future the role of PD-L1.
Expert opinion 2: Dr. Lukas Käsmann
STS represent a heterogeneous group of mesenchymal malignancies and nearly 50% of all patient suffering recurrences after complete resection. TIME may help us to differentiate immune cell populations which may serve as prognostic factors or biomarkers or even represent potential therapeutic targets. From my perspective targeting TAMs could be the most promising component of the TIME. Based on the macrophage subtype, TAM could be either immune-stimulatory (M1) with pro-tumor activities as well as immune-suppressive (M2) with anti-tumor properties. Thus, TAM dysbalance is often correlated with poor prognosis and therapy resistance, including radio-/chemo- and immunotherapies. I believe a deeper understanding of the TAMs in the TIME may provide new therapy opportunities and guidance for synergistic treatment approaches.
When it comes to the treatment of advanced unresectable STS, especially USRCS, it remains difficult given the heterogeneity in immunogenic features of histologic subtypes and varied responses to ICIs due to underlying primary or acquired resistance. How to evaluate and balance the effect and toxicity of combining ICI and immune-sensitizing agents [such as tyrosine kinase inhibitors (TKIs), cytotoxic chemotherapy, oncolytic viruses and etc.] to overcome underlying resistance mechanisms within sarcomas and the TME?
Expert opinion: Dr. Lukas Käsmann
A strong cooperation of preclinical researchers and physicians is needed in order to improve the understanding of resistance mechanisms as well as to balance treatment-related side effects of combined treatment approaches (e.g., TKIs, cytotoxic chemotherapy, oncolytic viruses, etc.). Evaluation of treatment response should be performed with the latest evaluation criteria e.g., RECIST 1.1 and iRECIST in order to compare different immunotherapy trials.
How should this patient be treated after disease progression?
Expert opinion 1: Dr. Massimiliano Bassi
I believe that a possible step in case of progression disease could be a re-biopsy in order to assess the new molecular and TIME path.
Expert opinion 2: Dr. Lukas Käsmann
Disease progression including local or distant progression should be discussed in a multi-disciplinary tumor board including surgeons, medical oncologist, pulmonologist, radiologist, pathologists and radiation oncologists. A new biopsy should be evaluated.
Local treatment including aggressive surgery may be considered in non-metastatic locally recurrent disease. Pulmonary metastasectomy in patients with relapsed disease and isolated lung metastases is controversial but could be performed based on individual decision-making. Reirradiation considering the dose constraints of the previous thoracic irradiation may be feasible. Oligo-progression could be treated based on location and tumor burden with local ablative radiotherapy. Palliative chemotherapy or radiotherapy should be interdisciplinary discussed after multisite progression.
Acknowledgments
We would like to thank Gang Yuan (Institute of Thoracic Oncology and Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, China) for support in providing language help. The authors also appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This work was supported by the 1·3·5 Project for Disciplines of Excellence Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2020HXFH046).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-572/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-572/coif). LL serves as an unpaid editorial board member of Translational Lung Cancer Research from July 2022 to June 2023. LK receives honoraria from AMGEN. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of West China Hospital, Sichuan University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gronchi A, Miah AB, Dei Tos AP, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2021;32:1348-65. [Crossref] [PubMed]
- von Mehren M, Kane JM, Agulnik M, et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:815-33. [Crossref] [PubMed]
- Kallen ME, Hornick JL. From the ashes of "Ewing-like" sarcoma: A contemporary update of the classification, immunohistochemistry, and molecular genetics of round cell sarcomas. Semin Diagn Pathol 2022;39:29-37. [Crossref] [PubMed]
- Le Loarer F, Baud J, Azmani R, et al. Advances in the classification of round cell sarcomas. Histopathology 2022;80:33-53. [Crossref] [PubMed]
- Antonescu CR, Owosho AA, Zhang L, et al. Sarcomas With CIC-rearrangements Are a Distinct Pathologic Entity With Aggressive Outcome: A Clinicopathologic and Molecular Study of 115 Cases. Am J Surg Pathol 2017;41:941-9. [Crossref] [PubMed]
- Kao YC, Owosho AA, Sung YS, et al. BCOR-CCNB3 Fusion Positive Sarcomas: A Clinicopathologic and Molecular Analysis of 36 Cases With Comparison to Morphologic Spectrum and Clinical Behavior of Other Round Cell Sarcomas. Am J Surg Pathol 2018;42:604-15. [Crossref] [PubMed]
- Ludwig K, Alaggio R, Zin A, et al. BCOR-CCNB3 Undifferentiated Sarcoma-Does Immunohistochemistry Help in the Identification? Pediatr Dev Pathol 2017;20:321-9. [Crossref] [PubMed]
- Sánchez Heras AB, López Rodríguez A, Pastor Borgoñón M, et al. Undifferentiated small cell tumor of the thoracopulmonary region. An Med Interna 1991;8:448-50. [PubMed]
- Richards R, Jour G, Tafe LJ, et al. Primary Pulmonary Round Cell Sarcomas: Multiple Potential Pitfalls for the Pathologist. Int J Surg Pathol 2022; Epub ahead of print. [Crossref] [PubMed]
- Tang L, Niu X, Wang Z, et al. Anlotinib for Recurrent or Metastatic Primary Malignant Bone Tumor: A Multicenter, Single-Arm Trial. Front Oncol 2022;12:811687. [Crossref] [PubMed]
- Kyriazoglou A, Gkaralea LE, Kotsantis I, et al. Tyrosine kinase inhibitors in sarcoma treatment. Oncol Lett 2022;23:183. [Crossref] [PubMed]
- Kelly CM, Antonescu CR, Bowler T, et al. Objective Response Rate Among Patients With Locally Advanced or Metastatic Sarcoma Treated With Talimogene Laherparepvec in Combination With Pembrolizumab: A Phase 2 Clinical Trial. JAMA Oncol 2020;6:402-8. [Crossref] [PubMed]
- Gordon EM, Chua-Alcala VS, Kim K, et al. SAINT: Results of an expanded phase II study using safe amounts of ipilimumab (I), nivolumab (N), and trabectedin (T) as first-line treatment of advanced soft tissue sarcoma [NCT03138161]. J Clin Oncol 2020;38:abstr 11520.
- Han Q, Liu S, Cui Z, et al. Case Report and Literature Review: Diagnosis, Tailored Genetic Counseling and Cancer Prevention for a Locally Advanced dMMR/MSI-H/TMB-H Lung Cancer Patient With Concurrent Lynch Syndrome Mediated by a Rare PMS2 Splicing Variant (c.1144+1G>A). Front Genet 2022;12:799807. [Crossref] [PubMed]
- Matissek KJ, Onozato ML, Sun S, et al. Expressed Gene Fusions as Frequent Drivers of Poor Outcomes in Hormone Receptor-Positive Breast Cancer. Cancer Discov 2018;8:336-53. [Crossref] [PubMed]
- Kim JH, Megquier K, Thomas R, et al. Genomically Complex Human Angiosarcoma and Canine Hemangiosarcoma Establish Convergent Angiogenic Transcriptional Programs Driven by Novel Gene Fusions. Mol Cancer Res 2021;19:847-61. [Crossref] [PubMed]
- Eulo V, Van Tine BA. Immune checkpoint inhibitor resistance in soft tissue sarcoma. Cancer Drug Resist 2022;5:328-38. [Crossref] [PubMed]
- Wilky BA. Immune checkpoint inhibitors: The linchpins of modern immunotherapy. Immunol Rev 2019;290:6-23. [Crossref] [PubMed]
- Hosokawa S, Takebayashi S, Mineta H, et al. Undifferentiated sarcoma of the maxillary sinus: report of a rare case in an adult. Auris Nasus Larynx 2009;36:92-5. [Crossref] [PubMed]
- Kim TK, Herbst RS, Chen L. Defining and Understanding Adaptive Resistance in Cancer Immunotherapy. Trends Immunol 2018;39:624-31. [Crossref] [PubMed]
- Gutiérrez-Jimeno M, Alba-Pavón P, Astigarraga I, et al. Clinical Value of NGS Genomic Studies for Clinical Management of Pediatric and Young Adult Bone Sarcomas. Cancers (Basel) 2021;13:5436. [Crossref] [PubMed]


